Abstract
Context
Previous studies have not examined the ability of multiple preconception biomarkers, considered together, to improve prediction of gestational diabetes mellitus (GDM).
Objective
To develop a preconception biomarker risk score and assess its association with subsequent GDM.
Design
A nested case-control study among a cohort of women with serum collected as part of a health examination (1984 to 1996) and subsequent pregnancy (1984 to 2009). Biomarkers associated with GDM were dichotomized into high/low risk.
Setting
Integrated health care system.
Participants
Two controls were matched to each GDM case (n = 256 cases) on year and age at examination, age at pregnancy, and number of pregnancies between examination and index pregnancy.
Main Outcome Measure
GDM.
Results
High-risk levels of sex hormone-binding globulin (SHBG; <44.2 nM), glucose (>90 mg/dL), total adiponectin (<7.2 μg/mL), and homeostasis model assessment-estimated insulin resistance (>3.9) were independently associated with 2.34 [95% confidence interval (CI): 1.50, 3.63], 2.03 (95% CI: 1.29, 3.19), 1.83 (95% CI: 1.16, 2.90), and 1.67 (95% CI: 1.07, 2.62) times the odds of GDM and included in the biomarker risk score. For each unit increase in the biomarker risk score, odds of GDM were 1.94 times greater (95% CI: 1.59, 2.36). A biomarker risk score including only SHBG and glucose was sufficient to improve prediction beyond established risk factors (age, race/ethnicity, body mass index, family history of diabetes, previous GDM; area under the curve = 0.73 vs 0.67, P = 0.002).
Conclusions
The improved, predictive ability of the biomarker risk score beyond established risk factors suggests clinical use of the biomarker risk score in identifying women at risk for GDM before conception for targeted prevention strategies.
Keywords: biomarker, gestational diabetes, preconception, pre-pregnancy, risk score
Gestational diabetes mellitus (GDM) is a common pregnancy complication, affecting 5% to 9% of pregnancies [1], with important short- and long-term health consequences for a mother and her child. In the short term, GDM is associated with an increased risk of hypertensive disorders during pregnancy, cesarean delivery, and macrosomia [2]. In the long term, GDM is associated with an increased risk of diabetes and cardiovascular disease in the mother [3, 4] and obesity, glucose intolerance, and diabetes in her child [5, 6].
Lifestyle interventions starting in early pregnancy for prevention of GDM have had limited success [7], potentially because the pathogenic processes leading to GDM have already begun before pregnancy [8]. Pregnancy-induced insulin resistance acts as a metabolic stress test to unmask underlying metabolic dysfunction [9]. Women with GDM have pancreatic beta cell dysfunction, which contributes to the inability to increase insulin secretion adequately to compensate for pregnancy-induced insulin resistance [10]. The majority of women who develop GDM also enter pregnancy with chronic insulin resistance, which further limits the insulin response to pregnancy-induced insulin resistance [11]. Preconception care, involving optimization of a woman’s health before planning and conceiving a pregnancy, as recommended by the American Congress of Obstetricians and Gynecologists [12], provides the potential for identification of women at high risk of GDM before pregnancy. Preconception interventions in these women may be more successful than interventions beginning in early pregnancy [8]; therefore, a clear definition of the phenotype at high risk may increase the success of prevention strategies.
Whereas several biomarkers [i.e., total adiponectin [13], sex hormone-binding globulin (SHBG) [14], total high-density lipoprotein (HDL) [15, 16], low-density lipoprotein (LDL) peak diameter [15], and gamma-glutamyltransferase (GGT) [17]] measured before pregnancy have been individually associated with risk of GDM in studies by our group, previous studies have not examined the ability of multiple preconception biomarkers considered together, beyond established risk factors, to improve prediction of GDM. To the best of our knowledge, a preconception biomarker risk score has not yet been developed and assessed. The objective of this study was to create a composite preconception biomarker risk score using biomarkers that have previously been associated with GDM risk and assess the association of this risk score with subsequent GDM, above and beyond established risk factors.
1. Methods
A. Study Setting
This study was conducted within Kaiser Permanente Northern California (KPNC), an integrated health care delivery system that provides medical care for approximately one-third of the population in the San Francisco Bay area. KPNC members are representative of the underlying population of this region [18]. From 1984 to 1996, female KPNC members were invited to complete a comprehensive health examination [multiphasic health checkup (MHC)] upon enrollment. The MHC consisted of a clinic visit for the completion of questionnaires and clinical measurements, including a random blood draw [19]. An extra serum sample was collected and stored at −40°C for future use.
B. Study Design and Population
We conducted a nested case-control study within the larger cohort of women who participated in the MHC. All subsequent pregnancies and births to MHC women were identified in the KPNC hospitalization database and the Pregnancy Glucose Tolerance and GDM Registry, an active surveillance registry that annually identifies all pregnancies resulting in a livebirth or stillbirth among KPNC members [20]. Women diagnosed with diabetes before the index pregnancy were then identified in the KPNC Diabetes Registry [21] and excluded from the current study. Of the 27,743 women, 15 to 45 year olds who participated in the MHC from 1984 to 1996, 4098 subsequently became pregnant and gave birth before 31 December 2010; had questionnaire and clinical data, including serum samples, available; and were free of recognized diabetes. Women diagnosed with GDM were considered cases. Two controls were selected for each case from among women who did not meet the GDM case definition during the study period. This study was approved by the Institutional Review Board of the Kaiser Foundation Research Institute. We have previously published results on individual preconception biomarker associations with GDM risk from this study [13–15, 17].
C. GDM Case Definition
GDM cases (n = 267) met the following criteria: glucose values obtained during a standard 100-gram, 3-hour oral glucose tolerance test that met the Carpenter-Coustan plasma glucose thresholds for GDM in the laboratory database (n = 228) or a hospital discharge diagnosis of GDM in the electronic hospital discharge database for pregnancies occurring before 1994, when the electronic laboratory data became available (n = 39). Trained abstractors conducted a standardized medical chart review to confirm that all 267 identified cases had 100-gram, 3-hour oral glucose tolerance test glucose values that met the Carpenter-Coustan criteria for GDM [two or more values exceeding plasma glucose thresholds: fasting, 5.3 mM (95 mg/dL), 1 hour, 10.0 mM (180 mg/dL), 2 hour, 8.6 mM (155 mg/dL), 3 hour, 7.8 mM (140 mg/dL)]. After chart review, cases were excluded for random glucose >200 mg/dL at the time of the MHC examination (indicating prepregnancy diabetes, n = 6) or no indication of GDM during the index pregnancy among women identified using a hospital discharge diagnosis of GDM (n = 5). A total of 256 confirmed cases of GDM with valid biomarker measurements were included in this study.
D. Control Selection and Matching Criteria
Controls were randomly selected among women without an indication of GDM. Two controls were individually matched to each case on year of MHC serum collection date (± 3 months), age at MHC serum collection (± 2 years), age at delivery of the index pregnancy (± 2 years), and number of pregnancies between serum collection and the index pregnancy (0, 1, ≥2). Cases and controls were matched on year of MHC serum collection to assure approximately equal sample storage time to account for potential degradation in the quality of the serum over time. Age at MHC serum collection, age at delivery of the index pregnancy, and number of pregnancies between the MHC serum collection and the index pregnancy were included as matching variables to address potential differences in age and parity between cases and controls, as GDM is more common in older women and women with greater parity [22, 23]. Controls were excluded from the analysis if they had glucose values that met the Carpenter-Coustan criteria for GDM found during medical chart review (n = 5), had an abnormal screening glucose but no follow-up diagnostic glucose test (n = 5), or had one abnormal glucose value on the diagnostic glucose test, suggestive of mild GDM (n = 5). Of the 508 identified matched controls, 497 were included in this study.
E. Biomarkers
Serum glucose was measured using the hexokinase method by the KPNC regional laboratory at the time of the MHC. For all other biomarkers, stored serum samples were thawed, aliquoted, and transported in batches on dry ice to laboratories for biomarker assays. Dr. Peter Havel’s laboratory at the University of California, Davis, performed the measurement of insulin, total adiponectin, SHBG, and GGT. Insulin was measured by radioimmunoassay (Millipore, Burlington, MA), SHBG was measured by ELISA (Alpco, Salem, NH), and GGT was measured on a Polychem analyzer (MedTest DX, Cortlandt Manor, NY). The intra-assay and interassay coefficients of variability were <4.0% and <10% (insulin), <6.0% and <9.0% (total adiponectin), and 4.7% and 5.6% (GGT), respectively. Dr. Ronald Krauss’s laboratory at the Children’s Hospital Oakland Research Institute performed the measurement of LDL peak diameter and HDL.
Insulin resistance was calculated using the homeostasis model assessment-estimated insulin resistance [HOMA-IR; = (fasting glucose × fasting insulin)/22.5], where glucose was measured in millimoles per liter, and insulin was measured in microunits per milliliter.
F. Covariates
All covariates were assessed at the MHC examination. Body mass index (BMI; in kilograms per square meter) was calculated using measured height (measured by stadiometer) and weight (measured by balance beam scale) at the MHC examination. Race/ethnicity, family history of diabetes, alcohol consumption, smoking, and education were assessed by self-administered questionnaires.
G. Statistical Analyses
Descriptive statistics were calculated separately for cases and controls. Means and SDs were used to describe continuous variables. Frequencies and percentages were used to describe categorical variables.
Selected biomarkers were dichotomized into high/low risk based on the distribution of each biomarker in the controls. For total adiponectin, SHBG, LDL peak diameter, and total HDL, values, less than the lowest quartile cutpoint (<7.2 μg/mL for total adiponectin, <44.2 nM for SHBG, <228.8 Å for LDL peak diameter, <3403.6 nM for total HDL), were considered high risk. For GGT, glucose, and HOMA-IR, values greater than the highest quartile cutpoint (>24.5 U/L for GGT, >90 mg/dL for glucose, >3.9 for HOMA-IR) were considered high risk. To determine which biomarkers to include in the biomarker risk score, high-risk indicator variables for each biomarker were included in a single, mutually adjusted, conditional logistic regression model. The model was also run adjusted for a priori-identified potential confounders: BMI at MHC examination (kilograms per square meter), race/ethnicity (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic), family history of diabetes (yes, no), alcohol use at MHC examination (yes, no, unknown), smoking at MHC examination (never, former, current, unknown), and education at MHC examination (≤12 years, 13 to <16 years, ≥16 years, unknown). All statistically significant biomarkers in this model were included in the biomarker risk score.
To create an unweighted biomarker risk score, the number of high-risk indicators that were significantly associated with GDM in the multivariable model was summed for each participant. To create a weighted biomarker risk score, a conditional logistic regression model, including all statistically significant biomarkers and all potential confounders listed above, was fit. Each high-risk indicator variable was multiplied by the coefficient for that biomarker from this model and summed for each participant. The formula for the weighted risk biomarker score for each participant was the following: high-risk SHBG × 0.7844 + high-risk glucose indicator × 0.7379 + high-risk adiponectin indicator × 0.6804 + high-risk HOMA-IR indicator × 0.4860.
Conditional logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for each unit increase in the unweighted and weighted biomarker risk score. The unweighted and weighted biomarker risk score was categorized into four categories, according to the distributions of the risk scores in the controls. Scores of zero were grouped into one reference category, and nonzero scores were categorized according to tertiles. Models were adjusted for a priori-identified potential confounders: BMI at MHC examination (kilograms per square meter), race/ethnicity (Non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic), family history of diabetes (yes, no), alcohol use at MHC examination (yes, no, unknown), smoking at MHC examination (never, former, current, unknown), and education at MHC examination (≤12 years, 13 to <16 years, ≥16 years, unknown). A model without the biomarker score, including established risk factors and potential confounders, was run to compare associations of biomarker scores with established risk factors for GDM. This model was adjusted for age at MHC ≥35 years, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander), overweight/obesity at MHC, family history of diabetes, alcohol use at preconception examination (yes, no, unknown), smoking at preconception examination (never, former, current, unknown), and education at preconception examination (≤12 years, 13 to <16 years, ≥16 years, unknown). Previous GDM was omitted from this model, as no controls had a history of GDM.
The relative incremental-predictive value of the unweighted and weighted continuous biomarker risk scores beyond established risk factors for GDM recommended by the American College of Obstetricians and Gynecologists [24] and the American Diabetes Association [25] [age at MHC examination ≥ 35 years old, high-risk race/ethnicity (non-Hispanic black, Hispanic, or Asian/Pacific Islander), prepregnancy (as assessed at MHC examination) overweight/obesity (BMI ≥ 25 kg/m2), family history of diabetes, and previous GDM (as assessed through review of the medical record)] was assessed using receiver-operating-characteristic curve analysis for matched case-control studies [26]. The relative incremental-predictive value of sequential unweighted biomarker scores, created starting with the biomarker most strongly associated with GDM and adding one biomarker based on decreasing strength of association, was also assessed. The area under the curve (AUC) for GDM risk prediction models, including unweighted and weighted biomarker risk scores, in addition to established risk factors for GDM, was compared by leave-one-out cross-validation using DeLong’s test. Select models were also compared using net reclassification improvement to measure improvement in classification of participants into risk categories based on predicted probabilities from the models (low risk = 0% to 30%, moderate risk = 31% to 60%, high risk = 61% to 100%) [27]. All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
2. Results
On average, women who developed GDM had a higher BMI at MHC examination (26.0 vs 23.7 kg/m2) and gained more weight from the MHC examination to their index pregnancy (8.9 vs 4.4 kg) than women who did not develop GDM (Table 1). Women who developed GDM were also more likely to be Asian/Pacific Islanders or Hispanic, to be multiparous at the time of the MHC, have a family history of diabetes, and not report alcohol use at the MHC examination than women who did not develop GDM.
Table 1.
Characteristics of Case and Control Participants at the Preconception Examination
|
|
GDM Cases (n = 256) |
Controls (n = 497) |
|---|---|---|
| Means (SD) | Means (SD) | |
| Age, y | 28.2 (5.5) | 28.4 (5.2) |
| Age at delivery, y | 35.4 (5.1) | 35.1 (4.9) |
| Time between preconception examination and delivery, y | 7.1 (4.4) | 6.7 (4.4) |
| BMI, kg/m2 | 26.0 (6.5) | 23.7 (4.6) |
| Weight change from preconception examination to pregnancy, kg | 8.9 (9.9) | 4.4 (8.2) |
| HOMA-IR index | 4.1 (3.5) | 2.9 (2.9) |
| Glucose, mg/dL | 89.6 (13.5) | 83.6 (8.3) |
| Insulin, μU/mL | 25.8 (28.6) | 17.5 (16.7) |
| Total adiponectin, μg/mL | 7.7 (3.5) | 10.6 (4.4) |
| SHBG, nM | 57.7 (45.1) | 79.7 (58.5) |
| GGT, U/L | 28.0 (21.7) | 22.4 (16.6) |
| LDL peak diameter, Å | 230.6 (5.6) | 232.0 (4.7) |
| Total HDL, nM | 4180.4 (1524.9) | 4650.8 (1605.5) |
| n (%) | n (%) | |
| Race/ethnicity | ||
| Non-Hispanic white | 50 (20) | 186 (37) |
| Non-Hispanic black | 91 (36) | 184 (37) |
| Asian/Pacific Islander | 80 (31) | 84 (17) |
| Hispanic | 35 (14) | 43 (9) |
| Nulliparous | 142 (34) | 278 (66) |
| Number of pregnancies between preconception examination and delivery | ||
| 0 | 182 (71) | 349 (70) |
| 1 | 61 (24) | 124 (25) |
| 2 | 11 (4) | 23 (5) |
| 3 | 2 (1) | 1 (0) |
| Family history of diabetes | 151 (59) | 192 (39) |
| History of GDM | 12 (5) | 0 (0) |
| Alcohol use | ||
| Yes | 149 (58) | 346 (70) |
| No | 74 (29) | 81 (16) |
| Unknown | 33 (13) | 70 (14.1) |
| Smoking | ||
| Current | 38 (15) | 61 (12) |
| Former | 37 (15) | 92 (19) |
| Never | 150 (59) | 277 (56) |
| Unknown | 31 (12) | 67 (14) |
| Education | ||
| ≤12 y | 74 (29) | 119 (24) |
| 13 to <16 y | 85 (33) | 157 (32) |
| ≥16 y | 92 (36) | 214 (43) |
| Unknown | 5 (2) | 7 (1) |
From the mutually adjusted model, high-risk levels of SHBG, glucose, total adiponectin, and HOMA-IR were each associated with greater odds of GDM (Table 2). Women in the lowest quartile (high risk) of SHBG had 2.34 times greater odds of GDM (95% CI: 1.50, 3.63) compared with women in the highest three quartiles (low risk) of SHBG. Women in the highest quartile (high risk) of glucose had 2.03 times greater odds of GDM (95% CI: 1.29, 3.19) compared with women in the lowest three quartiles (low risk) of glucose. Women in the lowest quartile (high risk) of total adiponectin had 1.83 times greater odds of GDM (95% CI: 1.16, 2.90) compared with women in the three highest quartiles (low risk) of total adiponectin. Women in the highest quartile (high risk) of HOMA-IR had 1.67 times greater odds of GDM (95% CI: 1.07, 3.62) compared with women in the lowest three quartiles (low risk) of HOMA-IR. High-risk levels of LDL peak diameter, GGT, and total HDL were not associated with odds of GDM (OR = 1.33, 95% CI: 0.84, 2.11; OR = 1.24, 95% CI: 0.80, 1.93; and OR = 1.04, 95% CI: 0.67, 1.60, respectively). SHBG, glucose, total adiponectin, and HOMA-IR were included in the unweighted and weighted biomarker risk scores.
Table 2.
Mutually Adjusted Associations of Biomarkers Assessed at the Preconception Examination with GDM
| Model 1a |
Model 2b |
||
|---|---|---|---|
| Biomarker |
High Risk |
OR (95% CI) | OR (95% CI) |
| SHBG | <44.2 nM | 2.74 (1.84, 4.09) | 2.34 (1.50, 3.63) |
| Glucose | >90 mg/dL | 2.05 (1.37, 3.08) | 2.03 (1.29, 3.19) |
| Total adiponectin | <7.2 μg/mL | 2.01 (1.34, 3.01) | 1.83 (1.16, 2.90) |
| HOMA-IR index | >3.9 | 1.76 (1.17, 2.64) | 1.67 (1.07, 2.62) |
| LDL peak diameter | <228.8 Å | 1.27 (0.84, 1.94) | 1.33 (0.84, 2.11) |
| GGT | >24.5 U/L | 1.43 (0.97, 2.13) | 1.24 (0.80, 1.93) |
| Total HDL | <3403.6 nM | 1.15 (0.77, 1.72) | 1.04 (0.67, 1.60) |
Model 1 is mutually adjusted for all biomarkers (high risk/low risk) listed.
Model 2 is additionally adjusted for BMI at preconception examination (kilograms per square meter), race/ethnicity (Non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic), family history of diabetes (yes, no), alcohol use at preconception examination (yes, no, unknown), smoking at preconception examination (never, former, current, unknown), and education at preconception examination (≤12 y, 13 to <16 y, ≥16 y, unknown).
For each one unit increase in the unweighted biomarker risk score (having high-risk levels of one additional biomarker), odds of GDM were 1.94 times greater (95% CI: 1.59, 2.36) after adjustment for potential confounders (Table 3). Women with an unweighted biomarker risk score of one had a 1.87 times greater odds of GDM (95% CI: 1.13, 3.10) compared with women with an unweighted biomarker risk score of zero. Women with an unweighted biomarker risk score of three or four had a 9.84 times greater odds of GDM (95% CI: 4.88, 19.9) compared with women with an unweighted biomarker risk score of zero.
Table 3.
Associations of the Preconception Biomarker Score and Established Risk Factors With GDM
| n Cases | n Controls | OR (95% CI) | |
|---|---|---|---|
| Established risk factors | |||
| Age at preconception examination ≥35 y olda | 26 | 60 | 0.38 (0.12, 1.18) |
| Race/ethnicitya | |||
| Non-Hispanic white | 50 | 186 | Reference |
| Non-Hispanic black | 91 | 184 | 1.46 (0.93, 2.29) |
| Asian/Pacific Islander | 80 | 84 | 3.00 (1.81, 4.97) |
| Hispanic | 35 | 43 | 2.68 (1.41, 5.07) |
| Overweight/obesity (BMI ≥ 25 kg/m2) at preconception examinationa | 123 | 140 | 2.81 (1.90, 4.14) |
| Family history of diabetesa | 151 | 192 | 2.12 (1.51, 2.97) |
| Preconception biomarker scores | |||
| Unweighted scoreb,c (continuous) | 256 | 497 | 1.94 (1.59, 2.36) |
| Unweighted scoreb,c (categorical) | |||
| Quartile 1 (0) | 42 | 209 | Reference |
| Quartile 2 (1) | 66 | 170 | 1.87 (1.13, 3.10) |
| Quartile 3 (2) | 77 | 78 | 5.04 (2.85, 8.90) |
| Quartile 4 (3–4) | 71 | 40 | 9.84 (4.88, 19.9) |
| P for trend | <0.0001 | ||
| Weighted scorec (continuous) | 256 | 497 | 2.70 (2.02, 3.62) |
| Weighted scorec (categorical) | |||
| Quartile 1 (0) | 55 | 209 | Reference |
| Quartile 2 (0.49–0.74) | 37 | 123 | 1.61 (0.91, 2.84) |
| Quartile 3 (0.78–1.22) | 58 | 80 | 2.93 (1.69, 5.08) |
| Quartile 4 (1.27–2.69) | 119 | 85 | 7.78 (4.30, 14.1) |
| P for trend | <0.0001 |
Model is adjusted for age at preconception examination ≥ 35 y, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander), overweight/obesity at preconception examination, family history of diabetes, alcohol use at preconception examination (yes, no, unknown), smoking at preconception examination (never, former, current, unknown), and education at preconception examination (≤12 y, 13 to <16 y, ≥16 y, unknown). Previous GDM was omitted from the model, as no controls had a history of GDM.
Score includes adiponectin, SHBG, HOMA-IR, and glucose.
Model is adjusted for BMI at preconception examination (kilograms per square meter), race/ethnicity (Non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic), family history of diabetes (yes, no), alcohol use at preconception examination (yes, no, unknown), smoking at preconception examination (never, former, current, unknown), and education at preconception examination (≤12 y, 13 to <16 y, ≥16 y, unknown). A separate model was used for each biomarker score.
Each additional unit of the weighted biomarker risk score was associated with 2.70 times greater odds of GDM (95% CI: 2.02, 3.62). Women with a weighted biomarker risk score in the highest quartile had 7.78 times greater odds of GDM (95% CI: 4.30, 14.1) compared with women with a weighted biomarker risk score of zero. Established GDM risk factors were associated with up to three times greater odds of GDM (OR range = 0.38 to 3.00), with the strongest established risk factor Asian race/ethnicity.
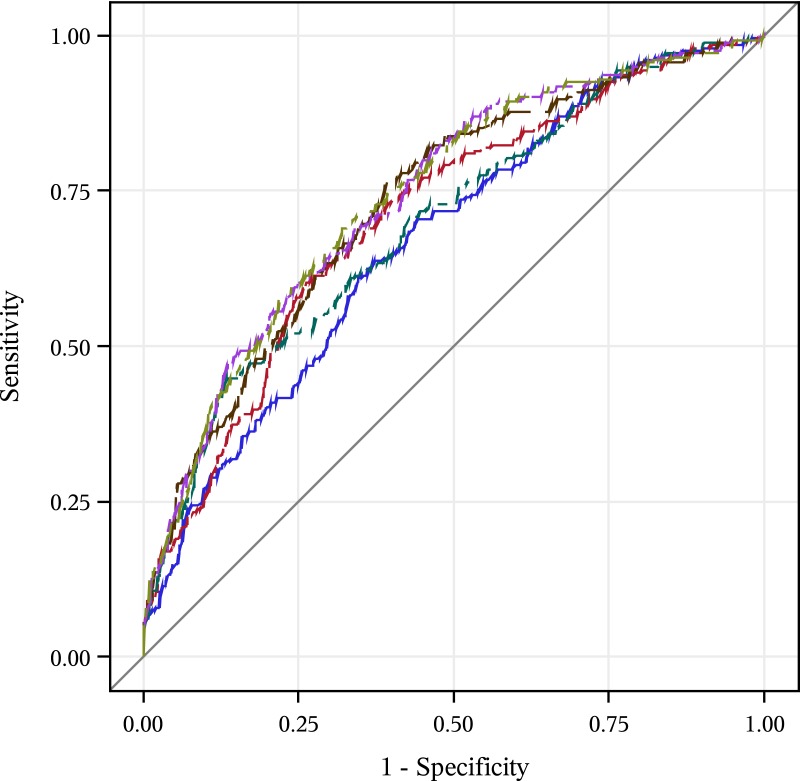
In receiver-operating-characteristic curve analyses, the continuous unweighted biomarker risk score had a relative incremental-predictive value beyond established risk factors for GDM (AUC = 0.74 vs 0.67; P value for comparison <0.0001)(Table 4). The weighted biomarker risk score did not have a relative incremental-predictive value over the unweighted risk score (AUC = 0.74 vs 0.74; P value for comparison = 0.09). An unweighted biomarker score, including only SHBG, increased relative incremental-predictive value beyond established risk factors for GDM (Fig. 1; AUC = 0.71 vs 0.67; P value for comparison = 0.02). An unweighted biomarker score, including only glucose, increased relative incremental-predictive value beyond established risk factors for GDM by a similar amount (AUC = 0.70 vs 0.67; P value for comparison = 0.02). An unweighted score, including SHBG and glucose, further increased the relative incremental-predictive value (AUC = 0.73; P value for comparison = 0.01). Addition of adiponectin and HOMA-IR to the unweighted biomarker score did not statistically significantly increase the relative incremental-predictive value (AUC = 0.74 for both; P for comparison = 0.14 and 0.98, respectively).
Table 4.
AUC for Predictive Value for GDM Risk
| Leave-One-Out, Cross-Validated AUC | P Value for AUC Comparison | |
|---|---|---|
| Model 1: established risk factorsa | 0.67 | |
| Model 2: established risk factorsa + SHBG | 0.71 | Model 2 vs 1: 0.02 |
| Model 3: established risk factorsa + glucose | 0.70 | Model 3 vs 1: 0.02 |
| Model 4: established risk factorsa + score with SHBG and glucose | 0.73 | Model 4 vs 1: 0.002 Model 4 vs 2: 0.01 |
| Model 5: established risk factorsa + score with SHBG, glucose, and adiponectin | 0.74 | Model 5 vs 1: <0.0001 Model 5 vs 4: 0.14 |
| Model 6: established risk factorsa + score with SHBG, glucose, adiponectin, and HOMA-IR | 0.74 | Model 6 vs 1: <0.0001 Model 6 vs 5: 0.98 |
Age at preconception examination ≥ 35 y, high-risk race/ethnicity (non-Hispanic black, Hispanic, Asian/Pacific Islander), overweight/obesity at preconception examination, family history of diabetes, and previous GDM.
Figure 1.
Leave-one-out, cross-validated, incremental-predictive value of incremental preconception biomarker score for prediction of GDM (blue, Model 1: established risk factors; red, Model 2: established risk factors + SHBG; green, Model 3: established risk factors + score with glucose; brown, Model 4: established risk factors + score with SHBG and glucose; purple, Model 5: established risk factors + score with SHBG, glucose, and adiponectin; yellow-green, Model 6: established risk factors + score with SHBG, glucose, adiponectin, and HOMA-IR).
3. Discussion
In this study, a preconception biomarker risk score, consisting of SHBG, glucose, total adiponectin, and HOMA-IR—measured, on average, 7 years before pregnancy—was associated with greater risk of GDM. A preconception biomarker risk score, including only SHBG and glucose, improved predictive ability for GDM beyond established risk factors, resulting in 18% of GDM cases (47 cases) being reclassified into a higher-risk category and 5% of controls (25 controls) being reclassified into a lower-risk category (net reclassification index = 0.23). In total, including a biomarker score with SHBG and glucose, this resulted in improved prediction for 72 women in our data. Addition of adiponectin and HOMA-IR to the preconception risk score did not further increase the predictive ability. This finding is of clinical relevance, as it suggests that a preconception biomarker risk score may identify a group of high-risk women who may otherwise not be identified as high risk for GDM using established risk factors. Identification of high-risk women before pregnancy may allow for preconception interventions to reduce the risk of GDM in future pregnancies.
Previous studies by our group have identified preconception biomarkers that were independently associated with increased GDM risk, including total adiponectin [13], SHBG [14], GGT [17], and total HDL [15] and lower LDL peak diameter [15]. When we included these biomarkers in a multivariable model in the current study, only SHBG, glucose, total adiponectin, and HOMA-IR were independently associated with GDM and thus, included in the risk score. The consideration of more than one biomarker in a risk score is more strongly associated with risk of future GDM than individual biomarkers or established GDM risk factors, with women with three or four biomarkers with high-risk levels at almost 10 times greater odds of GDM than women with no biomarkers with high-risk levels. Only the Coronary Artery Risk Development in Young Adults (CARDIA) study has also examined individual preconception biomarkers for GDM. The CARDIA study found that higher glucose and insulin levels and lower HDL levels (measurements of SHBG and adiponectin were not available in CARDIA), measured 3 years before pregnancy, were independently associated with 2.4 to 4.7 times greater odds of subsequent GDM after adjusting for established GDM risk factors [16]; however, a risk score was not evaluated, and the predictive ability of the biomarkers beyond established risk factors for GDM (receiver operating characteristic curves) was not assessed.
Few studies have considered multiple biomarkers together for GDM prediction, and these studies are limited to biomarkers assessed during pregnancy. A study by White et al. [28] assessed pregnancy biomarkers for GDM prediction in a high-risk cohort of 1303 obese, pregnant women. Addition of adiponectin, SHBG, glucose, hemoglobin A1c, triglycerides, and fructosamine, measured in the second trimester to a GDM prediction model, including age, previous GDM, family history of diabetes, systolic blood pressure, and anthropometric measures, improved the predictive value of the model (AUC = 0.77 vs 0.71). In a nested case-control study of 80 GDM cases and 300 controls attending hospital-based clinics in the United Kingdom, adiponectin and SHBG, measured at 11 to 13 weeks gestation, improved prediction of GDM beyond maternal risk factors, including age, BMI, race/ethnicity, family history of diabetes, history of GDM, and history of delivery of a macrosomic neonate when added to a predictive model (AUC = 0.84 vs 0.79) [29].
In contrast to the results of these previous studies that measured biomarkers during pregnancy, our use of a preconception risk score establishes temporality, and our results suggest that pathophysiological changes related to glucose homeostasis, adipocyte function, and sex hormone balance, which may lead to GDM, are also present years before pregnancies and are not a consequence of pregnancy-induced physiologic and hormonal changes in women who develop GDM. This suggests that women at high risk for GDM can be identified before pregnancy (during preconception care) for preconception interventions, which may be more effective than interventions beginning in early pregnancy for GDM prevention [8].
Our results suggest that a high-risk level of preconception SHBG may be a stronger predictor of future GDM than glucose and insulin resistance, as measured by HOMA-IR. An unweighted biomarker score, including SHBG and glucose, increased AUC by 3% and resulted in 13% of GDM cases (33 cases) being reclassified into a higher-risk category and 3% of controls (14 controls) being reclassified into a lower-risk category compared with a model, including glucose only (net reclassification index = 0.16). In total, including SHBG, in addition to glucose, in the biomarker score resulted in improved prediction for 47 women in our data. In women, lower levels of SHBG are a marker of a more androgenic hormonal profile that has been associated with increased risk of type 2 diabetes and insulin resistance [30–32], suggesting that SHBG may have functions contributing to glucose homeostasis and insulin resistance, in addition to its primary role of regulating and transporting sex steroids [31]. The role of SHBG in glucose homeostasis and GDM risk should be explored further.
Our results also suggest that high-risk levels of adiponectin before pregnancy are associated with future GDM but do not improve prediction of GDM beyond glucose and SHBG. Adiponectin is an adipokine with insulin-sensitizing effects [33, 34].
Our study has several strengths, including measurement of serum biomarkers before pregnancy to identify biological changes associated with GDM that precede pregnancy, complete GDM case ascertainment within our cohort, and consideration of multiple candidate biomarkers that have previously been individually associated with GDM in our study population. In addition, the population in our study was representative of the KPNC population and distributions of biomarkers in controls and in the general population were similar [35]. However, several limitations should be considered when interpreting our results. We did not have information available on other potential GDM risk factors, including measures of body fat mass, diet, and physical activity, which may impact associations. We were not able to validate the incremental-predictive value of our preconception biomarker score in a separate cohort. However, in the absence of a separate validation cohort, we used a computational approach (leave-one-out cross-validation) to replicate a validation dataset. Approximately one-half of the serum samples in our study were nonfasting (<6 hours since last food intake; 43% of cases, 47% of controls). Results of sensitivity analyses limited to women who were fasting at serum collection were similar to the main results [36], although independent associations of several biomarkers were no longer statistically significant. Associations of the preconception biomarker score with GDM were generally stronger in women who were fasting at serum collection; however, AUC values were similar to main results.
In conclusion, a preconception biomarker risk score, including SHBG, glucose, adiponectin, and HOMA-IR—measured, on average, 7 years before pregnancy—was associated with future GDM risk; however, a biomarker score, including only SHBG and glucose, may be sufficient to improve substantially the predictive ability for GDM beyond established GDM risk factors. Lifestyle interventions in early pregnancy to prevent GDM have had limited success [7], potentially because the pathogenic processes leading to GDM have already begun before pregnancy [8], as suggested by our finding. Future studies are needed to validate our preconception biomarker risk score in a separate cohort to support use of this risk score to identify women with high risk for future GDM. Future research should also include cost-benefit analyses, as SHBG is not a routinely measured biomarker in primary care. Identification of women at high risk for future GDM before pregnancy would allow for preconception interventions to attempt to alter the modifiable pathophysiologic pathways underlying the altered biomarker levels that we identified here.
Acknowledgments
Financial Support: This study was funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01-HD-065904 to M.M.H. S.E.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1T32DK11668401. S.F.E. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant K01DK105106.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- GDM
gestational diabetes mellitus
- GGT
gamma-glutamyltransferase
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment-estimated insulin resistance
- KPNC
Kaiser Permanente Northern California
- LDL
low-density lipoprotein
- MHC
multiphasic health checkup
- OR
odds ratio
- SHBG
sex hormone-binding globulin
References and Notes
- 1. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis. 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ovesen PG, Jensen DM, Damm P, Rasmussen S, Kesmodel US. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. A nation-wide study. J Matern Fetal Neonatal Med. 2015;28(14):1720–1724. [DOI] [PubMed] [Google Scholar]
- 3. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. [DOI] [PubMed] [Google Scholar]
- 4. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, Hu FB, Manson JE, Zhang C. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fraser A, Lawlor DA. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep. 2014;14(5):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–346. [DOI] [PubMed] [Google Scholar]
- 7. Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2017;11:CD010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond). 2015;39(4):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. [DOI] [PubMed] [Google Scholar]
- 10. Buchanan TA. Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(3):989–993. [DOI] [PubMed] [Google Scholar]
- 11. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–916. [DOI] [PubMed] [Google Scholar]
- 12. American College of Obstetricians and Gynecologists ACOG Committee Opinion number 313, September 2005. The importance of preconception care in the continuum of women’s health care. Obstet Gynecol. 2005;106(3):665–666. [DOI] [PubMed] [Google Scholar]
- 13. Hedderson MM, Darbinian J, Havel PJ, Quesenberry CP, Sridhar S, Ehrlich S, Ferrara A. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care. 2013;36(12):3930–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hedderson MM, Xu F, Darbinian JA, Quesenberry CP, Sridhar S, Kim C, Gunderson EP, Ferrara A. Prepregnancy SHBG concentrations and risk for subsequently developing gestational diabetes mellitus. Diabetes Care. 2014;37(5):1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, Hedderson MM. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol Metab. 2016;101(7):2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. Am J Epidemiol. 2010;172(10):1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sridhar SB, Xu F, Darbinian J, Quesenberry CP, Ferrara A, Hedderson MM. Pregravid liver enzyme levels and risk of gestational diabetes mellitus during a subsequent pregnancy. Diabetes Care. 2014;37(7):1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon N. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2009 California Health Interview Survey. Oakland, CA: Kaiser Permanente Division of Research; 2012. [Google Scholar]
- 19. Collen M. Multiphasic Health Testing Services. New York: John Wiley & Sons; 1978. [Google Scholar]
- 20. Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103(3):526–533. [DOI] [PubMed] [Google Scholar]
- 21. Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20(9):1396–1402. [DOI] [PubMed] [Google Scholar]
- 22. Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG. 2012;119(3):276–282. [DOI] [PubMed] [Google Scholar]
- 23. Egeland GM, Skjaerven R, Irgens LM. Birth characteristics of women who develop gestational diabetes: population based study. BMJ. 2000;321(7260):546–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Committee on Practice Bulletins—Obstetrics ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131(2):e49–e64. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 26. Pepe MS, Fan J, Seymour CW. Estimating the receiver operating characteristic curve in studies that match controls to cases on covariates. Acad Radiol. 2013;20(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pencina MJ, D'Agostino RB Sr., D'Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172; discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 28. White SL, Lawlor DA, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, Robson SC, Sattar N, Seed PT, Vieira MC, Welsh P, Whitworth M, Poston L, Pasupathy D; UPBEAT Consortium . Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLoS One. 2016;11(12):e0167846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135–141. [DOI] [PubMed] [Google Scholar]
- 30. Le TN, Nestler JE, Strauss JF III, Wickham EP III. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winters SJ, Gogineni J, Karegar M, Scoggins C, Wunderlich CA, Baumgartner R, Ghooray DT. Sex hormone-binding globulin gene expression and insulin resistance. J Clin Endocrinol Metab. 2014;99(12):E2780–E2788. [DOI] [PubMed] [Google Scholar]
- 32. Wang Q, Kangas AJ, Soininen P, Tiainen M, Tynkkynen T, Puukka K, Ruokonen A, Viikari J, Kähönen M, Lehtimäki T, Salomaa V, Perola M, Davey Smith G, Raitakari OT, Järvelin MR, Würtz P, Kettunen J, Ala-Korpela M. Sex hormone-binding globulin associations with circulating lipids and metabolites and the risk for type 2 diabetes: observational and causal effect estimates. Int J Epidemiol. 2015;44(2):623–637. [DOI] [PubMed] [Google Scholar]
- 33. Cheng KK, Lam KS, Wang B, Xu A. Signaling mechanisms underlying the insulin-sensitizing effects of adiponectin. Best Pract Res Clin Endocrinol Metab. 2014;28(1):3–13. [DOI] [PubMed] [Google Scholar]
- 34. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 35. Andreasson AN, Unden AL, Elofsson S, Brismar K. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. Am J Hum Biol. 2012;24(5):595–601. [DOI] [PubMed] [Google Scholar]
- 36. Badon SE. Data from: Supplemental Tables—A pre-pregnancy biomarker risk score improves prediction of future gestational diabetes. figshare. Dataset. 2018. Deposited 9 August 2018. https://figshare.com/articles/Supplemental_Tables-_A_pre-pregnancy_biomarker_risk_score_improves_prediction_of_future_gestational_diabetes/6953240. [DOI] [PMC free article] [PubMed]