Abstract
We present a case report of an early-onset drug reaction with eosinophilia and systemic symptoms (DRESS syndrome) induced by vemurafenib (BRAF inhibitor) in a middle-age man affected by a metastatic, BRAF mutant melanoma who was started on first-line metastatic treatment with vemurafenib and cobimetinib.
After initiating the treatment, the patient presented an extensive cutaneous rash with eosinophilia and renal impairment. Due the constellation of signs and symptoms, a diagnosis of DRESS syndrome was made which strongly contraindicated the reintroduction of vemurafenib due to its hypersensibility reaction. Thus, vemurafenib was stopped immediately, and we started corticoid treatment with clinical improvement.
Due to the contraindication to start vemurafenib again, after multidisciplinary view of the case and having balanced the risks and benefits, we successfully performed a switch to another BRAF inhibitor in a progressively ascending pattern, without any skin toxicity and with a good response of the metastatic melanoma.
Keywords: cancer - see oncology, skin cancer
Background
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a potentially lethal, rare form of drug-induced skin toxicity that usually appears many weeks after the trigger drug is started. In the last years, few cases of this entity related to cancer drug have been documented, even with immunotherapy.
This is a particularly interesting case due to the uncommon acute presentation and, because it was induced by vemurafenib. It highlights the importance of having a high index of suspicion of DRESS syndrome when a patient presents rash with eosinophilia and signs or symptoms of any organ impairment, specially renal or liver damage.
Because BRAF mutant melanoma is highly responsive to this therapy, this case shows that one important consequence of DRESS syndrome is that it is contraindicated to restart BRAF inhibitor, losing an important oncological treatment. On the other hand, a successful switch to another BRAF inhibitor was performed which allowed the patient to receive an oncological treatment with partial response of his metastatic melanoma that maintains nowadays.
Case presentation
A 50-year-old man was admitted to the hospital because of an episode of intestinal occlusion due to ileal intussusception. An intestinal resection was performed and two ulcerated intestinal metastases were found. The pathology study described metastases of melanoma with abdominal nodal involvement, the BRAF status was tested and a V600 mutation was found. Inguinal nodal involvement was also described when an abdominal CT was performed. No primary tumour was found after skin and eyes examination by dermatologist and ophthalmologist, respectively. A PET-CT (Positron emission tomography–computed tomography)was performed without evidence of more metastatic disease.
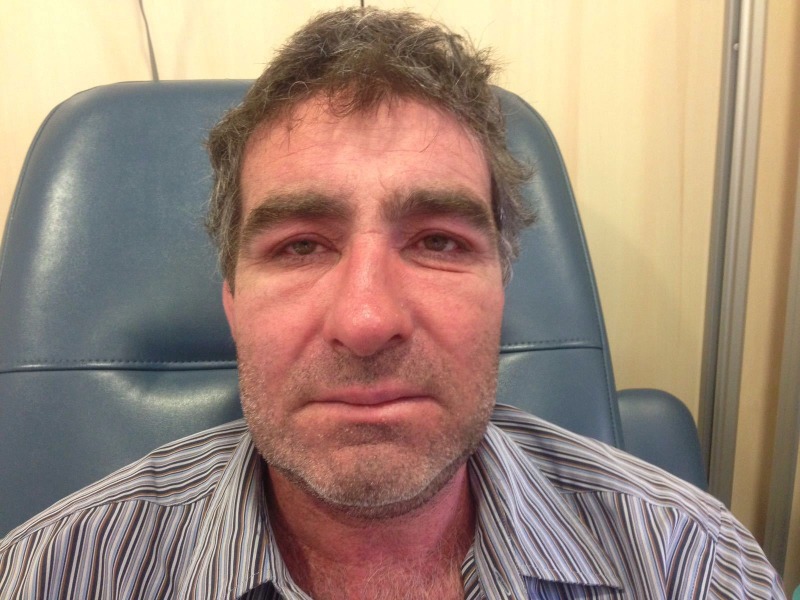
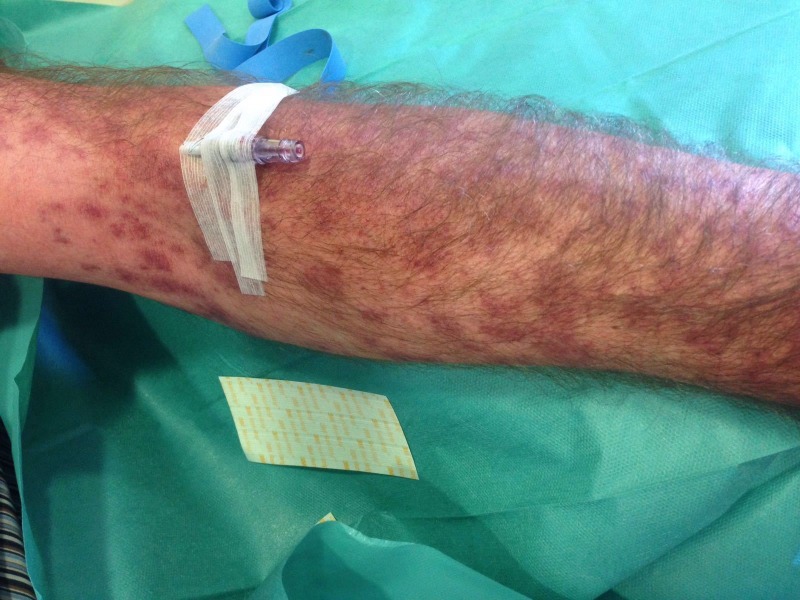
The patient was started on first-line metastatic melanoma treatment with a combination of vemurafenib 960 mg/12 hours and cobimetinib 60 mg/24 hours. Two weeks after starting the treatment, the patient was admitted to the emergency room with a 12-hour history of fever of 38.9°C, facial oedema and a G3 (CTCAE V.4.1) non-itchy morbilliform exanthem with palmoplantar, trunk, limbs (figure 1) and face involvement (figure 2) which affected more than 50% of body surface. The patient had not taken any other medications different from vemurafenib and cobimetinib.
Figure 1.
Acute facial oedema and cutaneous rash.
Figure 2.
Non-itchy morbilliform exanthem with extensive palmoplantar, trunk, limbs and face involvement.
Investigations
On physical examination, the patient had significant bilateral cervical and inguinal lymph nodes (which he did not have previously) without organomegaly. The blood sample showed leucocytosis of 25×109/L (normal range 4×109/L–11×109/L) without atypical lymphocytes. Alanine aminotransferase, aspartate aminotransferase and bilirubin were in normal range. There was eosinophilia 2.0×109/L (normal range 0–0.5×109/L) that later—during the following 5 days—reached up to 6.2×109/L) as well as deterioration of the renal function with creatinine level of 1.8 mg/dL (normal range 0.67–1.17 mg/dL). Serology for hepatitis viruses (including HAV, HBV, HCV, HEV and HDV) as well as real-time PCR for HHV 6 (Human Herpes Virus), EBV (Epstein-Barr Virus), CMV (cytomegalovirus) and antinuclear antibodies were negative twice, at the onset of DRESS syndrome and 8 weeks before when we repeat it to rule out viral reactivations. Serum IgG levels were not performed. Blood cultures were also negatives. A skin biopsy shown an intercellular epidermal oedema, moderate dermal lymphocytic infiltrate, erythrocyte extravasation and high eosinophil infiltrate with necrotic keratinocytes.
Faced with these findings and after evaluation by allergology and dermatology and despite of the negative results of viruses serologies, the episode was oriented as a definite case of DRESS syndrome induced by vemurafenib based on Registry of Severe Cutaneous Adverse Reactions (RegiSCAR) criteria (score of 7 points). Because this patient met enough criteria points for DRESS syndrome diagnosis, no further viral reactivation was tested.
Treatment
Supportive measures were provided and treatment with methylprednisolone intravenously 1 mg/kg was initiated. The treatment with vemurafenib was permanently stopped and it was recommended not to restart the same BRAF inhibitor due to the high risk of a severe hypersensitivity reaction. In the serological study, IgG resulted positive (high titres) for varicella zoster, rubella, measles and parvovirus.
After starting the corticoid treatment, the patient presented slow but progressive clinical improvement, the cutaneous lesions disappeared after 4 weeks of starting methylprednisolone. Once the cutaneous lesions were resolved, a tapering of corticosteroids dose was initiated. Patch test was not performed at the onset of DRESS syndrome and were performed 7 weeks later confirming at 72 and 96 hours reading strong positive reaction (++/+++) for vemurafenib. The cobimetinib patch test was negative. These results may exclude the possibility that this patient may have been desensitised to vemurafenib. For that reason, after an accurate evaluation by pharmacology, allergology and dermatology and due to the great benefit that vemurafenib and cobimetinib have shown in metastatic melanoma, it was decided to start another BRAF inhibitor; dabrafenib was initiated in a progressively ascending pattern: 75 mg/day for 3 days, thereafter 75 mg/12 hours dose for another 3 days and finally, a 150 mg/12 hours dose was reached. At these points, 2 mg/24 hours of trametinib was added, with excellent tolerance and without any relevant toxicity. Eosinophil count was 0.1×109/L (normal range 0–0.5×109/L) and renal function impairment had been resolved (creatinine 0.85 mg/dL, normal range 0.67–1.17 mg/dL) when dabrafenib was initiated.
Outcome and follow-up
Here, we report the case of a patient with metastatic melanoma presenting an early-onset DRESS syndrome caused by vemurafenib. Thanks to the multidisciplinary view—oncology, dermatology and allergology—this patient did not lose the opportunity to receive oncological treatment that had been withdrawal due to a serious adverse effect, and he was able to receive specific treatment for his cancer by switching to dabrafenib (BRAF inhibitor) getting a partial response. During the titration schedule, the patient came every 3 days to the hospital to perform physical examination. After that the visits were every 2 weeks for the first 6 months and actually every 6 weeks. Nowadays the patient is asymptomatic, with partial response of his disease, without any skin toxicity with the new treatment.
Discussion
DRESS syndrome, also known as drug-induced hypersensitivity syndrome, is a potentially lethal drug-induced hypersensitivity reaction that can involve different organs. The most frequent clinical features of this drug reaction include fever and trunk, palms, soles or mucosal rash. Other frequent clinical features are facial oedema, lymphadenopathy, haematological alterations and liver or renal dysfunction although brain, thyroid, lungs or pancreatic damage could also happen.1 Liver damage confers worse prognosis compared with other visceral alterations.2 Analytically highlights eosinophilia (90% of the cases) or atypical lymphocytosis. Eosinophilia may appear late, after the onset of symptoms. The first analytical alterations are frequently lymphopenia or lymphocytosis with atypical lymphocytes.1
This syndrome had been classically linked to allopurinol and carbamazepine as well as sulfasalazine, phenobarbital, lamotrigine and nevirapine. There are also cases of DRESS syndrome related to oncological treatments. Recently, a case report of DRESS syndrome due to ipilimumab and nivolumab treatment for a metastatic melanoma has been reported.3 On the other hand there are, in our concern, only few cases of DRESS syndrome induced by vemurafenib.4 5 The diagnosis is difficult due to the fact that clinical features may be incomplete. For that reason, the International RegiSCAR group has developed a scoring system trying to define accurately the DRESS syndrome and to differentiate it to other severe cutaneous reactions to drugs (toxic epidermal necrolysis and Stevens-Johnson syndrome).6 The diagnosis must include at least three clinical features as rash, fever, presence of lymph nodes, organ involvement and blood alterations such an eosinophilia, thrombocytopenia or lymphocytosis. There exist other scoring systems as the Japanese SCAR which adopted similar criteria but include measurement of anti-HHV-6 IgG titre.7
The pathophysiology of this entity still has not been fully defined. Toxic metabolites produced by a defect in the detoxification of some drugs, cause cellular damage or trigger an immune response.1 Pharmacogenetic studies shown that this syndrome is associated with both an exposure to specific drugs and pre-existing susceptibility. Otherwise, the reactivation of some viruses (HHV-6, HHV-7, EBV or CMV) has been associated clearly with this entity. In the study by Picard et al, about 76% of the patients with DRESS presented serologies compatible with reactivation of one of these viruses and also an important skin infiltration of cytotoxic CD8 lymphocytes for these viruses that could be also founded in the affected organs.8 Other theory propose that regulatory T-cells suppress activation of effector T-cells which cause a delay of the onset of DRESS syndrome and allows the progression of viral reactivation. Eventually, the regulatory T-cells become dysfunctional resulting in an autoimmune clinical picture marked by hypersensitivity.
The onset time of this entity usually takes place between 2 and 6 weeks after the drug is initiated, although there are reported cases of early-onset DRESS syndrome in relation to vemurafenib.9 Complete recovery of the rash usually takes place between 6 and 9 weeks after drug cessation, but in 20%–30% of patients rash progresses to exfoliating dermatitis. The mortality rate of this entity rounds 10% of the patients.10
The treatment consists of the immediate withdrawal of the drug suspicious of triggering the DRESS and initiating corticosteroid therapy, although it is not a fully validated treatment. Given the hypersensitivity reaction and the severity of the clinical case, the suspension of the drug is indispensable, so the search for new cancer therapy options will be indispensable to continue the oncological treatment.
In literature, there are reported few cases of severe cutaneous adverse effects (DRESS syndrome and toxic epidermal necrolysis) in which the switch from vemurafenib to another BRAF inhibitor such as dabrafenib could be done successfully, without described cutaneous reactions.9 11 It is also reported in literature a case of a successful desensitisation in a case of Stevens-Johnson syndrome due to vemurafenib in a patient who had a metastatic melanoma.12 Dabrafenib is approved as the second BRAF inhibitor in the treatment of metastatic melanoma and it presents a better profile of cutaneous toxicities and similar efficacy.
This case describes a successful treatment after switching to dabrafenib in a patient with DRESS syndrome induced by vemurafenib which has not been reported before. It also highlights the importance of performing a deep evaluation when drug-induced cutaneous reactions happen. The real success of this report is the resolution of DRESS syndrome and that the patient could receive specific target therapy for his metastatic disease. On the other hand, we cannot rule out that slow dosage titration schedule of dabrafenib may have served to desensitise to this BRAF inhibitor as has been reported in literature.13
On the other hand, vemurafenib is a BRAF inhibitor that was approved by the FDA (Food and Drug Administration) in 2011 for the treatment of metastatic unresectable melanoma with BRAF V600 mutation.14 This mutation is detected between 40% and 50% of cutaneous melanomas. Approximately, 90% of these mutations result in the substitution of glutamic acid for valine at codon 600 (BRAF V600E), although other activating mutations have been described (eg, BRAF V600K and BRAF V600R).15 This BRAF inhibitor has demonstrated a response rate of 50% in patients with advanced melanoma and shown a significant benefit both in progression-free survival and overall survival. The most frequent adverse effects of vemurafenib are cutaneous, including pruritus, photosensitivity, hyperkeratosis, squamous carcinomas, keratoacanthomas and maculopapular rash, which can affect from 36% to 68% of patients,9 although rarely serious.
However, serious adverse skin reactions have been reported with this drug, such as toxic epidermal necrolysis, Stevens-Johnson syndrome or DRESS syndrome.4 9 11 12 Acute generalised exanthematous pustulosis (AGEP) should be taken in consideration as a possible adverse effects of vemurafenib, even a possible overlap of AGEP and DRESS syndrome has been described.16
A retrospective French study that evaluated severe skin toxicities induced by vemurafenib in 131 patients reported 26% of grade 3–4 skin toxicity. Forty-four per cent of them underwent permanent vemurafenib discontinuation due to: maculopapular rash (12 patients), DRESS syndrome (1 patient), Steven-Johnson syndrome (1 patient) and one case of photosensitivity. Among patients with grade 3–4 toxicity (34 patients), 10 subsequently switched to dabrafenib which was well tolerated; skin toxicity only recurred in one patient. The patient who developed DRESS syndrome was moved to dabrafenib plus trametinib without relapse of DRESS nor any drug eruption17 per author communication.
On the other hand, safety profile of cobimetinib includes dermatological toxic effects but usually has decreased severity compared with that seen with vemurafenib. There are not DRESS syndrome, toxic epidermal necrolysis nor Stevens-Johnson syndrome cases reported with cobimetinib. Exceptionally, an erythema due to hypersensitivity reaction has been also described with cobimetinib, in a patient with melanoma who received it in combination with vemurafenib.18
Learning points.
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a potentially life-threatening drug-related entity with high morbimortality.
Vemurafenib is an important drug for metastatic melanoma with BRAF mutation with high response rates and benefit in overall survival with a well-known skin toxicity profile and only a few cases of severe skin adverse reactions that contraindicates the reintroduction of the drug. Vemurafenib has been associated with DRESS in several cases
Due to DRESS syndrome being a rare entity, the laboratory alterations and clinical features will be really important for suspecting the diagnosis.
The treatment of DRESS syndrome includes the immediate withdrawal of the inciting agent, supportive care and corticosteroids treatment to prevent a deeper worsening of the affected organs.
Switching to another BRAF inhibitor such as dabrafenib could be a potential option for these patients and allows them to continue the oncological therapy with a safety profile of skin adverse effects but this switch must be performed with caution.
Acknowledgments
The authors would like to thank to the patient and his family. JR wants to thank EM-C for her supervision in this medical case.
Footnotes
Contributors: JR drafted the initial manuscript. EM-C supervised and reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Descamps V, Ranger-Rogez S. DRESS syndrome. Joint Bone Spine 2014;81:15–21. 10.1016/j.jbspin.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Lens S, Crespo G, Carrión JA, et al. Severe acute hepatitis in the DRESS syndrome: Report of two cases. Ann Hepatol 2010;9:198–201. [PubMed] [Google Scholar]
- 3.Mirza S, Hill E, Ludlow SP, et al. Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res 2017;27:271–3. 10.1097/CMR.0000000000000326 [DOI] [PubMed] [Google Scholar]
- 4.Wenk KS, Pichard DC, Nasabzadeh T, et al. Vemurafenib-induced DRESS. JAMA Dermatol 2013;149:1242–3. 10.1001/jamadermatol.2013.5278 [DOI] [PubMed] [Google Scholar]
- 5.Johnson DB, Wallender EK, Cohen DN, et al. Severe cutaneous and neurologic toxicity in melanoma patients during vemurafenib administration following anti-PD-1 therapy. Cancer Immunol Res 2013;1:373–7. 10.1158/2326-6066.CIR-13-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med 2011;124:588–97. 10.1016/j.amjmed.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Criado PR, Avancini J, Santi CG, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a complex interaction of drugs, viruses and the immune system. Isr Med Assoc J 2012;14:577–82. [PubMed] [Google Scholar]
- 8.Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med 2010;2:46–62. 10.1126/scitranslmed.3001116 [DOI] [PubMed] [Google Scholar]
- 9.Munch M, Peuvrel L, Brocard A, et al. Early-onset vemurafenib-induced DRESS Syndrome. Dermatology 2016;232:126–8. 10.1159/000439272 [DOI] [PubMed] [Google Scholar]
- 10.Silva SA, Figueiredo MM, Carneiro L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). Rev Assoc Med Bras 2016;62:227–30. 10.1590/1806-9282.62.03.227 [DOI] [PubMed] [Google Scholar]
- 11.Jeudy G, Dalac-Rat S, Bonniaud B, et al. Successful switch to dabrafenib after vemurafenib-induced toxic epidermal necrolysis. Br J Dermatol 2015;172:1454–5. 10.1111/bjd.13522 [DOI] [PubMed] [Google Scholar]
- 12.Minor DR, Rodvien R, Kashani-Sabet M. Successful desensitization in a case of Stevens-Johnson syndrome due to vemurafenib. Melanoma Res 2012;22:410–1. 10.1097/CMR.0b013e3283573437 [DOI] [PubMed] [Google Scholar]
- 13.Chen CB, Wu MY, Ng CY, et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res 2018;10:1259–73. 10.2147/CMAR.S163391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809–19. 10.1056/NEJMoa1002011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gey A, Milpied B, Dutriaux C, et al. Severe cutaneous adverse reaction associated with vemurafenib: DRESS, AGEP or overlap reaction? J Eur Acad Dermatol Venereol 2016;30:178–9. 10.1111/jdv.12685 [DOI] [PubMed] [Google Scholar]
- 17.Peuvrel L, Quéreux G, Saint-Jean M, et al. Profile of vemurafenib-induced severe skin toxicities. J Eur Acad Dermatol Venereol 2016;30:250–7. 10.1111/jdv.13443 [DOI] [PubMed] [Google Scholar]
- 18.Patel U, Cornelius L, Anadkat MJ. MEK inhibitor-induced dusky erythema: characteristic drug hypersensitivity manifestation in 3 patients. JAMA Dermatol 2015;151:78–81. 10.1001/jamadermatol.2014.3207 [DOI] [PubMed] [Google Scholar]