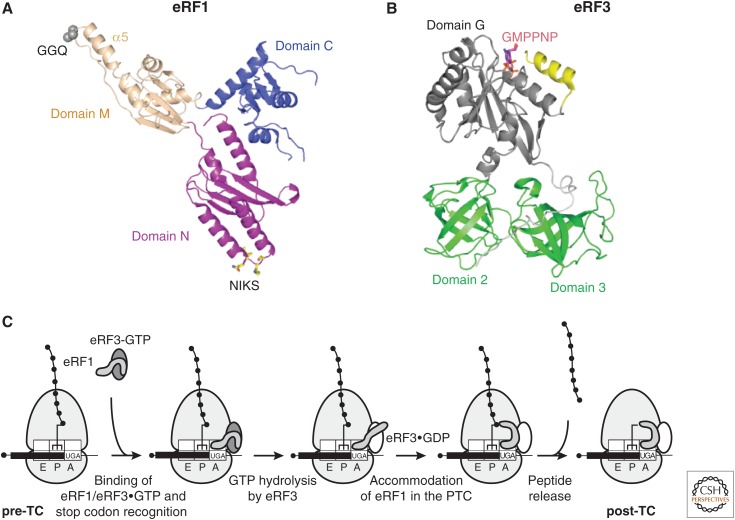
Figure 1.
Translation termination in eukaryotes. Ribbon representations of (A) human eRF1 (protein data bank [PDB]: 1DT9), with the Cα atoms of the GGQ motif in domain M and the NIKS motif in domain N shown in CPK and stick models, respectively, and (B) Schizosaccharomyces pombe eRF3 (amino acids 215–662, and thus lacking the nonconserved amino-terminal domain) (PDB: 1R5B), with bound GMPPNP shown in a stick model. (C) Outline of the termination process. The pretermination complex (pre-TC) contains peptidyl-transfer RNA (tRNA) in the P site. The eRF1/eRF3–guanosine triphosphate (GTP) complex binds to the A site of the pre-TC, and eRF1 recognizes the stop codon, which, with the +4 nucleotide, binds in a pocket formed by eRF1 and the 40S subunit. eRF1’s M domain dissociates from eRF3’s Switch I/Switch II elements, and after GTP hydrolysis by eRF3, accommodates in the peptidyl-transferase center (PTC), inducing peptide release. eRF1 and possibly eRF3-guanosine diphosphate (GDP) remain associated with post-termination complexes (post-TCs).