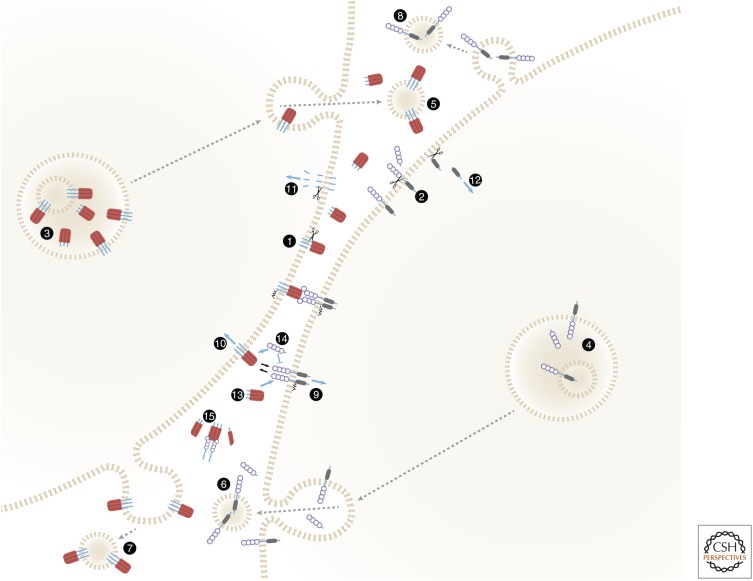
Figure 2.
Membrane-anchored and soluble forms of ligands and receptors of the tumor necrosis factor (TNF) families, and their functional roles. The figure shows an intriguing symmetry of the spectra of soluble and membrane-anchored forms attained by TNF family ligands and TNF/nerve growth factor (NGF) family receptors, and of the functions of these forms. Both ligands (1) and receptors (2) are proteolytically cleaved to yield soluble forms (Kriegler et al. 1988; Engelmann et al. 1990; Nophar et al. 1990; Black et al. 1997; Moss et al. 1997). The cleavage occurs constitutively or inducibly, either on the cell surface as illustrated (Black et al. 1997; Moss et al. 1997; Becker-Pauly and Rose-John 2013) or within the cell (Lopez-Fraga et al. 2001). (3,4) Some of the ligands (Gordon and Galli 1990; Bossi and Griffiths 1999; Koguchi et al. 2007) and receptors (Wang et al. 2003) accumulate within intracellular vesicles from which they are secreted in response to specific stimuli, thus supplementing either the cell-surface-expressed or the soluble pools. Some are released while anchored to membranes that might correspond either to exosomes that have accumulated in intracellular multivesicular bodies (5,6) or to microvesicles exfoliating from the cell surface (7,8) (Albanese et al. 1998; Martinez-Lorenzo et al. 1999; Islam et al. 2007). Binding of membrane-anchored ligands of the TNF family to their receptors, besides triggering receptor signaling (9), also triggers “reverse signaling” by the ligand molecules (10) (Stuber et al. 1995; Arens et al. 2004; Eissner et al. 2004; Grohmann et al. 2007; Kang et al. 2007; Sun et al. 2007; Juhasz et al. 2013). One mechanism contributing to this reverse signaling is intramembrane proteolytic cleavage, yielding ligand intracellular domain (ID) fragments that apparently mediate signaling following their translocation to the nucleus (Domonkos et al. 2001; Fluhrer et al. 2006; Friedmann et al. 2006; Kirkin et al. 2007) (11). Intramembrane cleavage that apparently contributes to signaling has also been reported for two receptors of the TNF/NGF family, nerve growth factor receptor (NGFR) and TNFR1 (Kanning et al. 2003; Kenchappa et al. 2006; Chhibber-Goel et al. 2016) (12). Accumulation of the soluble forms of both receptors and ligands interferes with the binding of membrane-bound forms to the ligands and the receptors, respectively, and can thus block signaling (13,14). To the extent that the soluble forms are incapable of triggering signaling, they may also interfere with signaling activation by their membrane-bound forms. However, the association of soluble receptors with soluble ligands also stabilizes the trimeric structures of the soluble ligands, so that they function not as mere inhibitors but rather as buffering agents. While decreasing the intensity of signaling activation, they also extend its duration (15) (Aderka et al. 1992; Eliaz et al. 1996).