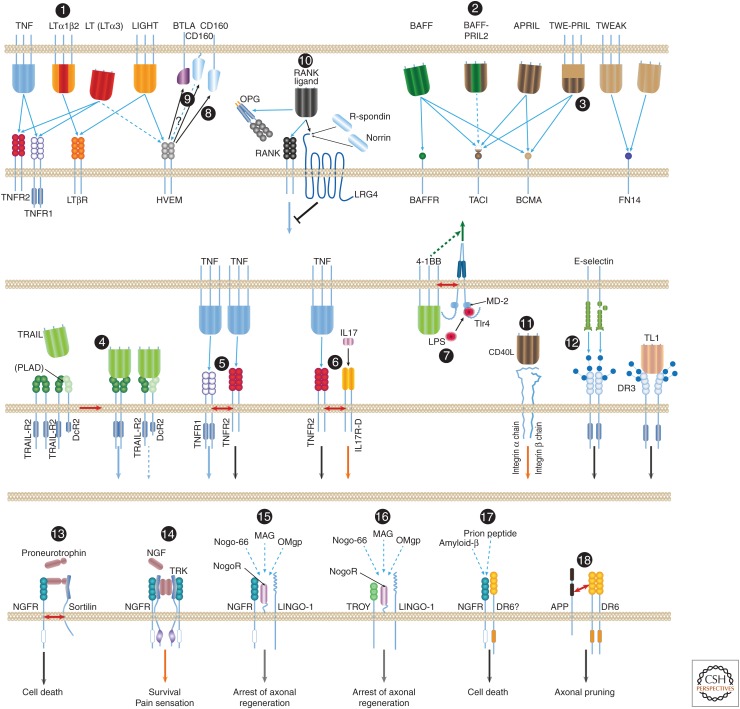
Figure 4.
Noncanonical associations of the tumor necrosis factor (TNF) ligand and the TNF/ nerve growth factor receptor (NGFR) families. Most of the interactions of ligands and receptors of the TNF ligand and TNF/NGFR families are known to occur between homotrimeric molecules of a particular ligand and molecules of a particular receptor. A few exceptions, however, are known: (1) Lymphotoxin β (LTβ) functions only within heterotrimers that it forms with lymphotoxin α (LTα). Whereas homotrimers of LT bind to the TNF receptors, the LTα1β2 heterotrimer binds to the LTβ receptor (LTβR) (Ware 2005). (2) B-cell-activating factor of the tumor necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) also form heterotrimers (so far discerned only in patients with autoimmune diseases), and these heterotrimers apparently activate only transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) (Roschke et al. 2002; Dillon et al. 2006; Schuepbach-Mallepell et al. 2015). (3) Although APRIL is constitutively shed and its trimers therefore occur only in soluble form, its gene also yields a fused joint splice variant with the neighboring TWEAK gene. The protein encoded by this transcript—TWE-PRIL—is anchored to the cell membrane (Pradet-Balade et al. 2002). (4) Association of molecules of TRAIL-R2 and the membrane-anchored truncated receptor DcR2 through their preligand assembly domain (PLAD) dictate association of TRAIL with the two receptors in mixed complexes wherein TRAIL-R2 signaling is suppressed by DcR2 (Clancy et al. 2005). (5) Molecules of the two receptors for TNF (TNFR1 and TNFR2) reportedly also associate on their binding to ligand. However, this association does not occur through binding of the two receptors to the same ligand molecule. In fact, the two receptor species are incapable of binding simultaneously to the same TNF molecule; molecules of the two receptors rather associate only after binding independently to distinct TNF molecules (Pinckard et al. 1997). (6) Molecules of TNFR2 reportedly also associate with molecules of IL-17 receptor D, a member of an unrelated cytokine-receptor family. This association, which occurs on triggering by the ligands of the two receptors, imposes assembly of aggregates of the two receptors and functional cooperation between them (Yang et al. 2015). (7) Another example of functional interaction with a structurally unrelated receptor is the association of the ligand for 4-1BB with a coexpressed TLR4–MD2 complex. This apparently occurs through the TMs of 4-1BBL and Tlr4 and the consequent potentiation of lipopolysaccharide (LPS)-induced Tlr4 signaling in a way that depends on the function of the intracellular domain (ID) of 4-1BBL but independently of the association of 4-1BBL with 4-1BB (Kang et al. 2007; Ma et al. 2013). (8) A different kind of noncanonical association is observed in the function of herpesvirus entry mediator (HVEM), a receptor of the TNF family. Besides its association with two TNF family ligands (with LIGHT, and [weakly] with LT homotrimers) it also binds to B- and T-lymphocyte attenuator (BTLA) and CD160, two cell-surface proteins of the immunoglobulin superfamily, thereby triggering inhibitory signaling by those two proteins (Shui et al. 2011). (9) Whether this association also triggers signaling by the ID of HVEM is not known. (10) RANKL, besides binding to RANK and to the soluble receptor osteoprotegerin (OPG), also binds to the seven-transmembrane (TM), leucine-rich repeat containing G protein-coupled receptor 4 (LGR4), which also serves as receptor for R-spondins and for Norrin. It thus triggers signaling antagonistic to that initiated by RANK (Luo et al. 2016). (11) Binding of soluble CD40L to integrin αIIbβ3 and to integrin α5β1 triggers signaling by those two membrane–protein complexes (Andre et al. 2002; Leveille et al. 2007). (12) Binding of the cell-adhesion lectin, E-selectin, to DR3-linked sialic-acid-linked sugar chains triggers signaling by DR3 (Porquet et al. 2011). Juxtaposition and activation of the two TRAIL receptors, DR4 and DR4, by TRAIL also depends, for a reason not yet clear, on glycosylation of these receptors (Wagner et al. 2007). The most elaborate known set of noncanonical associations is observed in the function of the NGFR, a receptor of the TNF/NGF family for which no ligand of the TNF family is known. NGFR contributes to signaling for different effects in response to different inducers through association with a series of different coreceptors. (13) It contributes to signaling for death in response to proneurotrophins, to which it binds in association with a sortilin family receptor. (14) When associating with a Trk tyrosine kinase receptor, apparently through the TMs and IDs of both receptors (Esposito et al. 2001), NGFR contributes to high-affinity binding of NGF, and signals for cell survival and for pain sensation. (15) NGFR is also found to form, through extracellular domain (ED) associations, a ternary complex with the glycosylphosphatidylinositol (GPI)-linked Nogo-66 receptor (NogoR) and the TM receptor LINGO-1. In response to myelin-associated inhibitory factors (a 66-amino-acid fragment of the oligodendrocyte-derived growth inhibitory protein Nogo, the oligodendrocyte myelin glycoprotein [OMgp] or the myelin-associated glycoprotein [MAG]), this complex signals for arrest of axonal regeneration following injury (Hempstead 2002; Chao 2003; Ibanez and Simi 2012). (16) In that complex, NGFR can be replaced by the orphan TNF family receptor TROY (Shao et al. 2005). (17) Finally, direct binding of amyloid-β to NGFR (Hempstead 2002; Chao 2003; Ibanez and Simi 2012), and probably also to a complex of NGFR and another orphan receptor of the TNF/NGF family, DR6 (Hu et al. 2013), reportedly triggers signaling for neuronal death. (18) DR6 was found to bind to a carboxyl-terminal region in the ED of the amyloid precursor protein (APP). APP and DR6 cooperate in the induction of axonal pruning. The mechanism underlying this cooperation is not clear, nor is it known whether the cooperation occurs between proteins expressed in the same cell, as illustrated in the figure, or in distinct cells (Olsen et al. 2014). BCMA, B-cell maturation antigen.