Abstract
A 12-yr-old normocalcemic female treated for a ruptured ovarian juvenile granulosa cell tumor at an outside hospital presented for exploratory laparotomy and gross surgical debulking of pelvic recurrence. Morphologically, the tumor was composed of sheets and nests of small blue cells forming cysts of various sizes and focal mucinous differentiation. Epithelial membrane antigen (EMA), patchy inhibin, and strong and diffuse p53 immunoreactivity were also observed. A revised diagnosis of mixed sex cord stromal tumor with heterologous elements was favored because of the inhibin immunoreactivity. Targeted next-generation sequencing of the tumor revealed a SMARCA4 c.1141C>T, p.Arg381Ter (NM_001128849.1) nonsense mutation and an in-frame 18-bp TP53 deletion (c.594_611del18, p.Gly199_Glu204del, NM_001126112.2). Cytogenetic analysis revealed a normal 46,XX karyotype, and OncoScan single-nucleotide polymorphism array analysis demonstrated copy-neutral loss of heterozygosity (CN-LOH) of 19p13.3-19p13.2 and mosaic CN-LOH of 17p13.3-p11.2 encompassing the SMARCA4 and TP53 loci, respectively. Subsequent germline SMARCA4 sequencing confirmed a heterozygous SMARCA4 p.Arg381Ter mutation. In lieu of the molecular findings, the diagnosis was amended to small cell carcinoma of the ovary, hypercalcemic type (SCCOHT). The patient was treated aggressively with paclitaxel, carboplatin, and bevacizumab. She received an autologous stem cell transplant but died 5 mo after SCCOHT diagnosis secondary to complications of the transplant. This case expands the morphologic, immunophenotypic, and genomic spectrum of SCCOHT and highlights how multimodal molecular analysis can assist with the diagnosis and clinical management of SCCOHT patients.
Keywords: ovarian neoplasm
INTRODUCTION
Highly aggressive small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is the most common undifferentiated ovarian malignancy in women <40 yr of age (mean age of 23.9 yr). In 1994, nearly a decade after the initial descriptions (Scully 1979; Dickersin et al. 1982), Young and colleagues published a case series of 150 SCCOHT patients that codified the clinicopathologic understanding of this entity (Young et al. 1994). Approximately two-thirds of patients with SCCOHT demonstrate increased serum calcium, and a majority of patients have extra-ovarian disease or distant metastases at presentation. Even when diagnosed at an early stage, patients with SCCOHT have poor survival. Therefore, an accurate diagnosis of SCCOHT is critical for appropriate clinical management.
SCCOHT is currently classified by the World Health Organization as a miscellaneous ovarian neoplasm (McCluggage et al. 2014). In the classic morphologic description, mitotically active high-grade small round blue tumor cells grow in nests, cords, or trabeculae with associated fluid-filled follicle-structures. Focal morphologic variations are common, and mucinous elements are detected in ∼10% of cases. A common diagnostic dilemma is distinguishing SCCOHT from sex cord stromal tumors—namely juvenile granulosa cell tumor (JGCT). However, patients with JGCTs typically have normal preoperative serum calcium, and JGCTs demonstrate inhibin positivity, whereas SCCOHT is uniformly negative. Strong p53 immunoreactivity is a feature of SCCOHT (McCluggage et al. 2004), and epithelial membrane antigen (EMA) is also preferentially expressed. However, EMA positivity has been described in some cases of JGCT (Witkowski et al. 2016).
In 2014, three separate groups identified germline and somatic SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator chromatin A4) mutations as the molecular hallmark of SCCOHT (Jelinic et al. 2014; Ramos et al. 2014; Witkowski et al. 2014). SMARCA4 encodes Brahma-related gene 1 (BRG1) which is a component of a multiprotein SWI/SNF ATP-dependent chromatin remodeling complex, and loss of BRG1 protein staining by immunohistochemistry is a sensitive and specific marker for this tumor (Karanian-Philippe et al. 2015; Conlon et al. 2016). Prior to this discovery, germline SMARCA4 mutations were described in a small percentage of pediatric patients with rhabdoid tumor predisposition syndrome, a tumor syndrome characterized by morphologically similar atypical teratoid/rhabdoid tumor (AT/RT) of the brain or malignant rhabdoid tumors of visceral organs that occur as a result of germline mutations in SMARCB1 (which encodes INI1, another component of the SWI/SNF complex) (Brennan et al. 2013; Witkowski and Foulkes 2015). Given their morphologic and molecular similarities (Fahiminiya et al. 2016), it has been proposed that SCCOHT be renamed “malignant rhabdoid tumor of the ovary,” and the diagnosis require molecular demonstration of SMARCA4 mutation or loss of BRG1 expression (Foulkes et al. 2014; Witkowski et al. 2016).
Herein we present the first reported case of a normocalcemic adolescent female with an SCCOHT with patchy inhibin positivity. Tumor-only-targeted next-generation sequencing (NGS) detected a SMARCA4 nonsense mutation, later confirmed to be of heterozygous germline origin, and an in-frame 18-bp TP53 deletion within the p53 DNA-binding domain. OncoScan single-nucleotide polymorphism (SNP) array revealed copy-neutral loss of heterozygosity (CN-LOH) of 19p13.3-p13.2 and 17p13.3-p11.2 (mosaic) containing the SMARCA4 and TP53 loci, respectively. This case expands the immunohistochemical profile of SCCOHT, highlights CN-LOH as a mechanism of biallelic inactivation in SCCOHT, underscores the utility of SNP array analysis to identify CN-LOH, and emphasizes the importance of comprehensive molecular diagnostics for the diagnosis, management, and follow-up testing of young women with high grade ovarian malignancies.
RESULTS
Clinical Presentation and Family History
A 12-yr-old female with a history of mild von Willebrand disease presented to an outside hospital in January 2016 with periumbilical abdominal pain. A detailed family history was noncontributory with no evidence of malignancy or abdominal disorders. Initial imaging revealed a 22-cm left ovarian tumor with spontaneous rupture, and emergency surgical debulking was performed. A diagnosis of JGCT was rendered, and she was treated with four cycles of etoposide, ifosamide, and cisplatin. Nine months after initial diagnosis, surveillance imaging revealed a pelvic recurrence. At the time of recurrence, serum CA-125 was elevated at 89 U/ml (normal 0–35 U/ml), but inhibin, AFP, HCG, and calcium levels were all within normal limits.
Histology and Immunohistochemistry
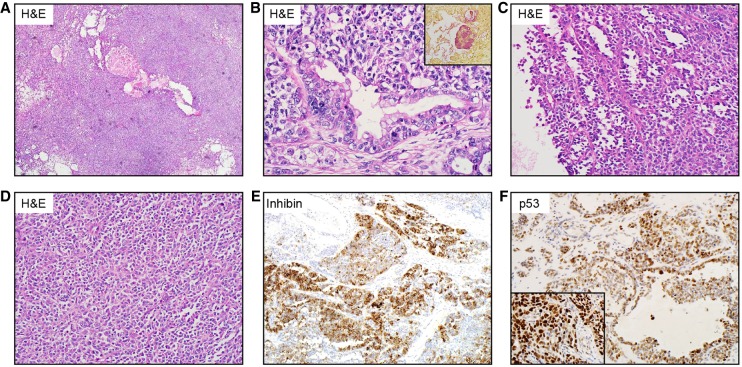
The pelvic mass was resected at our institution and submitted for pathologic examination. The recurrence was composed of sheets and nests of highly mitotically active small blue cells with variably sized cysts (Fig. 1A–D) and a minor component of heterologous mucinous differentiation (Fig. 1B), which stained positive for EMA and mucicarmine (Fig. 1B, inset). The tumor demonstrated WT1 and CD56 immunoreactivity, and inhibin (Fig. 1E) and calretinin staining were patchy and scattered. Certain areas of the tumor were strongly positive for p53 (Fig. 1F). Estrogen receptor showed equivocal staining, whereas progesterone receptor and Melan A were negative. Given the inhibin positivity, mucinous differentiation, and p53 staining, a diagnosis of mixed sex cord stromal tumor with heterologous elements was favored.
Figure 1.
(A) Low-power hematoxylin and eosin (H&E) section showing sheets and nests of highly mitotically active small blue cells with variably sized cysts. (B) A minor component of heterologous mucinous differentiation, which stained positive for mucicarmine (inset), is shown. Microcystic (C) and solid (D) regions of tumor. (E) Inhibin immunoreactivity seen in a subset of tumor cells. (F) Positive immunoreactivity for p53, with positive control (inset).
Molecular Studies
All molecular tests were clinically validated, insurance-reimbursable assays ordered by licensed healthcare providers. The results are summarized in Tables 1 and 2. The CLIA-certified Cancer Genomics, Cancer Cytogenetics, and Molecular Oncology laboratories at Texas Children's Hospital performed tumor sample testing of DNA mutations using the Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v.2 (Life Technologies) and structural and copy-number alterations using conventional cytogenetics (G-banding karyotype) and OncoScan FFPE tissue SNP array, respectively. A commercially available Molecular Intelligence Profile (MI Profile, Caris Life Sciences) was ordered to assess additional biomarkers for therapeutic decision support and clinical trials matching. SMARCA4 germline sequencing was performed at Prevention Genetics laboratory on DNA extracted from whole blood.
Table 1.
Variant table
| Gene | Chromosome | HGVS DNA reference | HGVS protein reference | Variant type | Predicted effect | dbSNP/dbVar ID | Genotype | ClinVar ID | Parent of origin | Observed effect (if shown to be different from predicted effect) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP53 | 17:7578238 (GRCh37) | NM_001126112.2 c.594_611del18 | NP_001119584.1 p.Gly199_Glu204del | Deletion, in-frame | Alters DNA binding domain, may be dominant negative | None | Not tested | None | Not tested | N/A | Mosaic copy-neutral LOH of TP53 locus |
| SMARCA4 | 19:11100015 (GRCh37) | NM_001128849.1 c.1141C>T | NP_001122321.1 p.Arg381Ter | Substitution, nonsense | Loss of function | None | Heterozygous germline | 470230 | Not tested | N/A | Copy-neutral LOH at SMARCA4 locus |
N/A, not applicable; LOH, loss of heterozygosity.
Table 2.
Summary of patient's clinical molecular testing results
| DNA sequencing | |||||||
|---|---|---|---|---|---|---|---|
| Gene | GRCh37/hg19 position | RefSeq RNA | Variant (CDS) | Variant (protein) | VAF (%) | Assay | Performing laboratory |
| TP53 (somatic) | Chr 17:7578238 | NM_001126112.2 | c.594_611del18 | p.G199_E204del | 25.9 | CHPv2 | TCH-CGL |
| 22 | MI Profile | Caris Life Sciencesa | |||||
| SMARCA4 (somatic) | Chr 19:11100015 | NM_001128849.1 | c.1141C>T | p.R381X | 88 | MI Profile | Caris Life Sciencesa |
| 88.8 | TCH-PST | TCH-CGL | |||||
| SMARCA4 (germline) | Chr 19:11100015 | NM_001128849.1 | c.1141C>T | p.R381X | Heterozygous | Germline seq | Prevention geneticsa |
| OncoScan SNP array analysis | |||||||
|---|---|---|---|---|---|---|---|
| Region | Chr Start (GRCh37/hg19) | Chr Stop (GRCh37/hg19) | Size (Mb) | Gene count | CN state | Array nomenclature | Performing laboratory |
| 17p13.3-p11.2 | Chr 17:400958 | Chr 17:20896038 | 20.5 | 388 | 2 | arr[hg19] 17p13.3p11.2 (400,958-20,896,038)x2 hmz mos | TCH-MOL |
| 19p13.3-p13.2 | Chr 19:247231 | Chr 19:12425269 | 12.2 | 389 | 2 | arr[hg19] 19p13.3p13.2 (247,231-12,425,269)x2 hmz | TCH-MOL |
| Conventional karyotyping | |||||||
|---|---|---|---|---|---|---|---|
| 46,XX[20] | TCH-CCL | ||||||
CHPv2, Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v.2; MI Profile, Molecular Intelligence Profile; Germline seq, germline sequencing; TCH-CGL, Texas Children's Hospital Cancer Genomics Laboratory; TCH-PST, Texas Children's Hospital Pediatric Solid Tumor Mutation Panel; TCH-MOL, Texas Children's Hospital Molecular Oncology Laboratory; TCH-CCL, Texas Children's Hospital Cancer Cytogenetics Laboratory.
aDenotes reference laboratory testing.
Karyotype and OncoScan SNP array
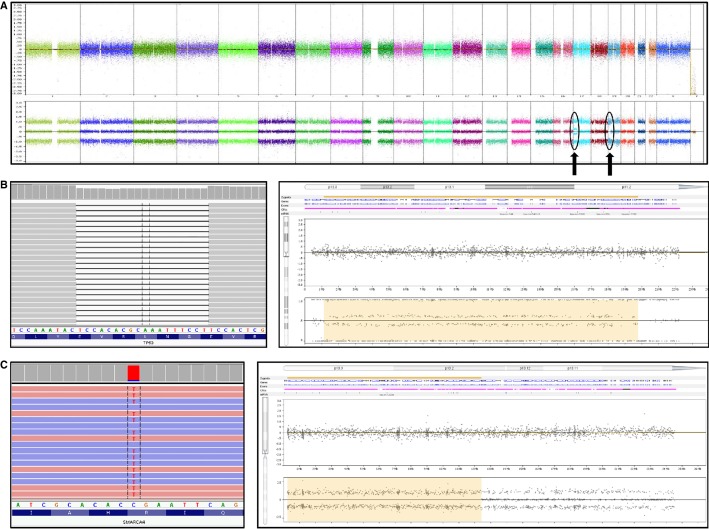
Conventional G-banding revealed a 46,XX[20] normal female karyotype (Table 1). OncoScan SNP array analysis of the same DNA used for targeted DNA mutation analysis (see below) revealed a diploid 46,XX array (Fig. 2A) with a 12.2-MB region of CN-LOH of Chromosome 19p13.3-13.2, which contained the SMARCA4 locus (Fig. 2B, right), and a 20.5-MB region of mosaic CN-LOH of chromosome bands 17p13.3-p11.2, which contained the TP53 locus (Fig. 2C, right).
Figure 2.
(A) OncoScan Nexus Copy Number 7.5 (Nexus) view showing a diploid 46,XX array with focal abnormalities in Chromosomes 17p and 19p (arrows). (B) Integrative Genomics Viewer (IGV) 2.3 view of TP53 c.594_611del18 in-frame deletion of 18 nt at 25.9% variant allele fraction (VAF) (left). Nexus view showing mosaic copy-neutral loss of heterozygosity (CN-LOH) of Chromosome 17p13.3-p11.2 including the TP53 locus (right; log2 ratio and B-allele frequency view). (C) IGV 2.3 view of orthogonal targeted next-generation sequencing confirmation of SMARCA4 c.1141C>T single-nucleotide variant at 88% VAF (left). Nexus view showing CN-LOH of Chromosome 19p13.3-19p13.2 including the SMARCA4 locus (right; log2 ratio and allele peak view).
Tumor DNA mutation analysis
Sections of formalin-fixed, paraffin-embedded (FFPE) tissue harboring ∼90% neoplastic nuclei by microscopic examination of a representative hematoxylin and eosin (H&E) slide were submitted for molecular analyses. The Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v2 (CHPv2, Life Technologies) sequences hotspot regions in 50 frequently mutated tumor suppressor genes and oncogenes, as described (Ballester et al. 2016). CHPv2 analysis revealed an 18-bp deletion in TP53 (c.594_611del18) predicted to encode an aberrant TP53 protein (p.Gly199_Glu204delGNLRVE, NM_001126112.2). The variant was detected in 1445 reads with total average coverage of 5572× yielding a variant allele fraction (VAF) of 25.9% (Fig. 2B, left) and confirmed by Sanger sequencing. The Caris MI Profile confirmed the TP53 deletion at a VAF of 22% and identified a SMARCA4 c.1141C>T p.Arg381Ter (NM_001128849.1) nonsense mutation at a VAF of 88%. No coverage data were available in the report. POLE mutations recently reported in SCCOHTs (Jelinic et al. 2016) were not detected by the Caris MI profile assay. Notably, SMARCA4 is not targeted by the CHPv2 assay (Supplemental Table S1).
For internal orthogonal confirmation, residual DNA tested in the CHPv2 assay was prepared for sequencing using an in-house 124 gene pediatric solid tumor mutation panel that was in clinical validation at the time of initial testing. The SMARCA4 c.1141C>T p.Arg381Ter variant was detected in 685 reads with a total coverage of 771× yielding a VAF of 88.8% similar to the Caris MI Profile test. An IGV2.3 pile-up view of NGS read data highlighting the SMARCA4 single-nucleotide variant is shown (Fig. 2C, left).
Germline SMARCA4 sequencing
A peripheral blood sample was sent to Prevention Genetics for “Ovarian Cancer and Rhabdoid Tumor Predisposition Syndrome testing via SMARCA4 NextGen Sequencing.” The results were positive for a heterozygous SMARCA4 c.1141C>T, p.Arg381Ter mutation. The average sequencing coverage of the entire SMARCA4 gene was 436× but no variant read coverage data were provided in the report.
Follow-up and Treatment
SMARCA4 mutation detection prompted the surgical pathologist to revise the final diagnosis to “high-grade ovarian tumor, consistent with ovarian small cell carcinoma of hypercalcemic type.” Once the germline origin of the mutation was confirmed, the patient's family was offered genetic counseling but they declined further testing and no additional family data were obtained. Given the revised diagnosis, the clinical team elected to pursue aggressive clinical management. After additional chemotherapy with bevacizumab, paclitaxel, and carboplatin, the patient received an autologous stem cell transplant. However, the patient passed away 5 mo after her SCCOHT diagnosis because of complications from the transplant.
DISCUSSION
We report the first case of an SCCOHT with patchy inhibin positivity; inhibin immunoreactivity has not been reported previously and is considered universally negative in this tumor type (McCluggage et al. 2004; Distelmaier et al. 2006). Tumor-only sequencing revealed a pathogenic SMARCA4 c.1141C>T, p.Arg381Ter nonsense mutation (NM_001128849.1) that was confirmed to be of heterozygous germline origin, and a TP53 c.594_611del18, p.Gly199_Glu204del (NM_001126112.2) predicted to encode a TP53 protein with an aberrant p53 DNA-binding domain. SNP array analysis identified CN-LOH of 19p13.3-19p13.2 and mosaic CN-LOH of 17p13.3-p11.2 resulting in biallelic inactivation of SMARCA4 and TP53, respectively. Collectively, this case expands the morphologic, immunophenotypic, and genomic spectrum of SCCOHT and highlights the utility of multimodal molecular analyses in high-grade malignant tumors of the ovary.
The histopathologic diagnosis of the high-grade ovarian neoplasm in this case was so challenging that three separate diagnoses (JGCT, mixed sex cord stromal tumor with heterologous elements, and SCCOHT) were all considered in the morphologic differential. Based on limited published literature, the patient's normocalcemic presentation (although not specific) and inhibin immunoreactivity were key factors in the original diagnoses; however, the correct diagnosis of SCCOHT was rendered only after molecular confirmation. The Caris MI Profile, although primarily requested for therapeutic decision planning, was selected in part because of its inclusion of SMARCA4 because this gene was not covered by the in-house CHPv2 targeted assay. Thus, MI Profile had the added advantage of potentially identifying a diagnostic variant of established clinical significance for an entity considered in the histopathologic differential. BRG1 immunohistochemistry, loss of which is specific for SCCOHT (Clarke et al. 2016), would have been a useful diagnostic adjunct but was unavailable at the time of the initial diagnostic workup. Additionally, Jelinic et al. (2016) reported loss of SMARCA2 IHC staining in 90% of SCCOHT cases tested, whereas only 2% (1/50) of other tumors lost SMARCA2 expression. Thus, SMARCA2 IHC may have also been a useful diagnostic adjunct in this case.
SCCOHTs present in a similar age group and demonstrate morphologic overlap with poorly differentiated sex cord stromal tumors. In a recent review by Witkowski et al. (2016), inhibin was considered consistently negative in SCCOHT and endorsed as a useful stain to distinguish SCCOHT (inhibin negative) from adult and juvenile GCTs (inhibin positive). A review of the literature yielded no reported cases of inhibin IHC staining in 19 cases of SCCOHT (McCluggage et al. 2004, Distelmaier et al. 2006). Thus, to our knowledge, this is the first reported case of an SCCOHT that showed patchy inhibin positivity. This case identifies an important potential diagnostic pitfall and broadens the immunophenotypic spectrum of this rare tumor.
BRG1, the protein encoded by the SMARCA4 gene, is a putative tumor suppressor and critical modulator of transcriptional regulation, replication, DNA repair, and recombination (Hendricks et al. 2004; Trotter and Archer 2008), and biallelic inactivation of SMARCA4 is required to abrogate BRG1 protein function. Witkowski et al. reported that for SCCOHTs harboring somatic or germline SMARCA4 loss of function mutations the second allele is inactivated by acquired LOH in ∼40% of cases (Witkowski et al. 2014). Notably in their cohort, LOH was the acquired second hit for one patient that harbored an identical germline SMARCA4 c.1141C>T, p.Arg381Ter mutation. SCCOHTs typically have noncomplex karyotypes with 19p loss reported as the only recurrent chromosomal alteration (Fahiminiya et al. 2016). Although the high VAF observed for the SMARCA4 p.Arg381Ter mutation was suggestive of LOH (88%), conventional karyotype analysis revealed a normal diploid karyotype. We reasoned that loss of the second allele, if present, was either below the limit of resolution of the karyotype or secondary to CN-LOH. Indeed, SNP array analysis confirmed CN-LOH as the “second hit” for the SMARCA4 locus.
Despite strong p53 immunoreactivity consistently reported as feature of SCCOHT as seen in this case (McCluggage et al. 2004), the molecular basis for TP53 inactivation in SCCOHT is not well established in the literature. We identified an 18-bp in-frame TP53 deletion expected to encode a mutant protein lacking 6 amino acids within the p53 DNA-binding domain. A review of the Catalog of Somatic Mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=TP53, accessed June 25, 2018) revealed no identical entries; however, more than 200 single-nucleotide variants or insertions/deletions affecting amino acids Gly199-Gly204 (NM_001126112.2) are reported. Thus, the predicted effect of this mutation coupled with mosaic CN-LOH of 17p13.3-p11.2 (including TP53) is suggestive of biallelic somatic TP53 inactivation and supported by the strong p53 immunoreactivity. Tissue was available only from the pelvic recurrence so we were unable to assess whether the TP53 mutation was present in the original tumor which is a limitation of this study. Notably, CN-LOH is not readily detected by conventional methodologies such as karyotyping, FISH, or comparative genomic hybridization array, and array analysis in the literature is limited (Gamwell et al. 2013). Therefore, additional investigation is warranted to determine the role for CN-LOH in TP53 inactivation in these tumors. Collectively, this case highlights the utility of SNP array analysis for detecting biallelic inactivation via CN-LOH in SCCOHTs with normal karyotypes.
Somatic mutation testing of a “JGCT/mixed sex cord stromal tumor with heterologous elements” performed for therapeutic indications ultimately led to a redefined pathologic diagnosis and unmasked an unknown hereditary germline associated with cancer predisposition. Tumor-only sequencing is performed for diagnostic, prognostic, or predictive indications and is often not intended to distinguish somatic from germline mutation events. In this case, ascribing germline or somatic origin to the mutations in the absence of a matched germline sample was not feasible. Testing a peripheral blood sample confirmed the heterozygous germline origin of the SMARCA p.Arg381Ter mutation, although we were unable to confirm the mode of inheritance or investigate the family further because of lack of consent, which is a limitation of the current study. Our case is another example of SCCOHT arising in the background of SMARCA4 germline mutation (Witkowski et al. 2014, 2016). Also, this case reiterates the principle that tumor-only sequencing, particularly in children, can unmask unexpected germline findings (Raymond et al. 2016).
In conclusion, we report the first case of an SCCOHT with patchy inhibin positivity in a normocalcemic adolescent female harboring germline SMARCA4 and somatic TP53 mutations with biallelic inactivation secondary to CN-LOH. Given the inherent diagnostic challenges of this entity, we favor that the diagnosis of “malignant rhabdoid tumor of the ovary (SCCOHT)” requires molecular confirmation, and we support the recommendation that tumor SMARCA4 sequencing or BRG1 immunohistochemistry be performed for all high-grade small round blue cell tumors of the ovary (Clarke et al. 2016; Witkowski et al. 2016). In addition, we show that SNP array offers superior resolution to the conventional karyotype when assessing for copy-number alterations in SCCOHT. Last, germline mutation testing should be performed when SMARCA4 mutations are detected by tumor-only sequencing in SCCOHT given the high frequency of germline SMARCA4 mutations (Ramos et al. 2014; Witkowski et al. 2014).
METHODS
Inhibin and p53 Immunohistochemistry
Immunohistochemistry was performed on 10% FFPE samples, cut on a microtome (3–5-µm thickness) and stained according to the standard protocols. The antibody initially used for Inhibin alpha was mouse anti-human antibody clone R1 (Catalog# PA0110, Leica). The inhibin alpha immunohistochemical stain was subsequently repeated using a new antibody batch of clone R1 (Catalog# PA0488, Leica). The antibody used for p53 was mouse monoclonal antibody clone DO7 (Catalog# MU239-UC, BioGenex).
Conventional Cytogenetics (G-Banding Karyotype)
Cytogenetic analysis was performed as previously described (Castro et al. 2016). Briefly, tumor tissue was minced and cultured in Dulbecco modified Eagle medium containing 15% fetal calf serum, 1% l-glutamine, and 1% penicillin-streptomycin and Primocin. Cultures were harvested, and G-banding was performed using standard protocols. Clonal chromosomal abnormalities identified by G-banding were described according to the International System for Human Cytogenetic Nomenclature (2016) in 20 metaphase cells interpreted.
OncoScan Single-Nucleotide Polymorphism Array
Genome-wide copy-number variation (CNV) and LOH profiling was performed on genomic DNA isolated from FFPE tumor tissue using the Affymetrix OncoScan FFPE Assay Kit. The assay was performed according to the manufacturer's protocol. CEL files generated from the array were analyzed using the OncoScan Console 1.3 software to create OSCHP files containing copy-number calls. Data analysis was performed using the Affymetrix CHAS 3.1 software and OncoScan Nexus Copy Number 7.5 software and aligned to the National Center for Biotechnology Information (NCBI) human build GRCh37/hg19 assembly. Chromosomal regions with copy-number variations of >50 kb and all regions of homozygosity (absence or copy-neutral) of >5 Mb were highlighted for manual evaluation by the pathologists (K.E.F. and D.H.L.-T.).
Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v.2 (50 Genes)
Genomic DNA was extracted from FFPE tumor sections containing ∼90% neoplastic nuclei. The Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v.2 (Life Technologies) sequence hotspot regions in 50 frequently mutated tumor suppressor genes and oncogenes (see Supplemental Table S1) as described (Ballester et al. 2016). The raw signal data were analyzed using Torrent Suite v.4.2 (Life Technologies) and variants called with the Torrent Variant Caller. Coverage metrics for the sequenced sample are provided in Table 3.
Table 3.
Sequencing metrics for Ion Torrent Cancer Hotspot Panel
| Total reads | 1,106,521 |
| Aligned reads | 1,073,874 |
| Percent reads on target | 98.80 |
| Minimum coverage (all targets) | 41× |
| Maximum coverage (all targets) | 11720× |
| Average coverage (all targets) | 4965× |
| Percent of target regions of ≥100× coverage | 99.61 |
Texas Children's Hospital Pediatric Solid Tumor Mutation Panel (124 Genes)
The TCH Pediatric Solid Tumor Mutation Panel (TCH-PST) is a custom-designed NGS panel tailored to detect point mutations and small indels in the entire coding regions and select noncoding regions of 124 genes involved in solid tumor malignancies of childhood (Supplemental Table S1). Genomic DNA was reextracted from additional FFPE tumor sections cut from the same tumor block used for the CHPv2 assay. Barcoded libraries were constructed from genomic DNA with the KAPA Biosystems HyperPlus kit followed by capture hybridization-based target enrichment using a custom-designed Roche NimbleGen SeqCap Target Enrichment probe set and sequencing on the Illumina MiSeq System. FASTQ files were aligned to the GRCh37 (hg19) reference human genome using Burrows-Wheeler Aligner (BWA) and NextGENe v2.4.1.2. Variant calling was performed using NextGENe v2.4.1.2 and Platypus v0.8.1 and variants were annotated with Variant Effect Predictor to produce a merged annotated variant list. Only single and multinucleotide variations and small insertions and deletions (<25 bp in size) in captured bases of the ROI were assessed; copy-number alterations and structural variations in genes were excluded. Coverage metrics for the sequenced sample are provided in Table 4.
Table 4.
Sequencing metrics for TCH Pediatric Solid Tumor Mutation Panel
| Total reads | 12,045,016 |
| Aligned reads | 10,949,032 |
| Unique reads passing filter | 8,844,034 |
| Percent reads on target | 77.62 |
| Maximum coverage (all targets) | 3367× |
| Average coverage (all targets) | 540× |
| Percent of target regions of ≥100× coverage | 97.04 |
| Percentage of bases of <100× coverage | 1.8 |
Reference Laboratory Testing
The Molecular Intelligence Profile (MI Profile) was performed at Caris Life Sciences. The MI Profile tests for molecular biomarkers using NGS for point mutation, indel, copy-number alterations, and fusion detection, and also uses pyrosequencing, immunohistochemistry, chromogenic in situ hybridization, and fragment length analysis for select genes. See Supplemental Table S1 for a complete list of the 637 biomarkers queried by the assay. SMARCA4 germline sequencing was performed at Prevention Genetics. Additional test information for the “Ovarian Cancer and Rhabdoid Tumor Predisposition Syndrome via the SMARCA4 Gene” is available at www.preventiongenetics.com.
ADDITIONAL INFORMATION
Data Deposition and Access
The p53 and SMARCA4 variants were deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV000804853 and SCV000804854, respectively. Per laboratory regulations, raw sequencing data were not released to public databases.
Ethics Statement
All testing performed was part of clinical patient management and not on a research basis. Per the Baylor College of Medicine Human Research Protections Manual and Institutional Review Board Procedures this work is IRB-exempt: “Case reports or case series describing interesting observations on three or fewer patients do not meet the definition of research as a systematic investigation designed to contribute to generalizable knowledge. Therefore the IRB does not review or approve such reports.”
Acknowledgments
The authors would like to thank all members of the clinical laboratories for their excellent technical assistance.
Author Contributions
M.P.D. conceived and drafted the manuscript. R.V. provided clinical patient management and contributed sections to the manuscript. D.H.L.-T. interpreted cytogenetics and SNP array data. A.R. interpreted sequencing data. N.P. performed histologic assessment and contributed sections to the manuscript draft. K.E.F. interpreted sequencing and array data and drafted the manuscript.
Funding
No external funding sources used.
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Ballester LY, Sarabia SF, Sayeed H, Patel N, Baalwa J, Athanassaki I, Hernandez JA, Fang E, Quintanilla NM, Roy A, et al. 2016. Integrating molecular testing in the diagnosis and management of children with thyroid lesions. Pediatr Dev Pathol 19: 94–100. [DOI] [PubMed] [Google Scholar]
- Brennan B, Stiller C, Bourdeaut F. 2013. Extracranial rhabdoid tumours: what we have learned so far and future directions. Lancet Oncol 14: e329–e336. [DOI] [PubMed] [Google Scholar]
- Castro E, Cortes-Santiago N, Ferguson LM, Rao PH, Venkatramani R, López-Terrada D. 2016. Translocation t(7;12) as the sole chromosomal abnormality resulting in ACTB-GLI1 fusion in pediatric gastric pericytoma. Hum Pathol 53: 137–141. [DOI] [PubMed] [Google Scholar]
- Clarke BA, Witkowski L, Ton Nu T, Shaw PA, Gilks CB, Huntsman D, Karnezis AN, Sebire N, Lamovec J, Roth LM, et al. 2016. Loss of SMARCA4 (BRG1) protein expression as determined by immunohistochemistry in small-cell carcinoma of the ovary, hypercalcaemic type distinguishes these tumours from their mimics. Histopathology 69: 727–738. [DOI] [PubMed] [Google Scholar]
- Conlon N, Silva A, Guerra E, Jelinic P, Schlappe BA, Olvera N, Mueller JJ, Tornos C, Jungbluth AA, Young RH, et al. 2016. Loss of SMARCA4 expression is both sensitive and specific for the diagnosis of small cell carcinoma of ovary, hypercalcemic type. Am J Surg Pathol 40: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickersin GR, Kline IW, Scully RE. 1982. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer 49: 188–197. [DOI] [PubMed] [Google Scholar]
- Distelmaier F, Calaminus G, Harms D, Sträter R, Kordes U, Fleischhack G, Göbel U, Schneider DT. 2006. Ovarian small cell carcinoma of the hypercalcemic type in children and adolescents. Cancer 107: 2298–2306. [DOI] [PubMed] [Google Scholar]
- Fahiminiya S, Witkowski L, Nadaf J, Carrot-Zhang J, Goudie C, Hasselblatt M, Johann P, Kool M, Lee RS, Gayden T, et al. 2016. Molecular analyses reveal close similarities between small cell carcinoma of the ovary, hypercalcemic type and atypical teratoid/rhabdoid tumor. Oncotarget 7: 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes WD, Clarke BA, Hasselblatt M, Majewski J, Albrecht S, McCluggage WG. 2014. No small surprise—small cell carcinoma of the ovary, hypercalcaemic type, is a malignant rhabdoid tumour. J Pathol 233: 209–214. [DOI] [PubMed] [Google Scholar]
- Gamwell LF, Gambaro K, Merziotis M, Crane C, Arcand SL, Bourada V, Davis C, Squire JA, Huntsman DG, Tonin PN, et al. 2013. Small cell ovarian carcinoma: genomic stability and responsiveness to therapeutics. Orphanet J Rare Dis 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks KB, Shanahan F, Lees E. 2004. Role for BRG1 in cell cycle control and tumor suppression. Mol Cell Biol 24: 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinic P, Mueller JJ, Olvera N, Dao F, Scott SN, Shah R, Gao J, Schultz N, Gonen M, Soslow RA, et al. 2014. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet 46: 424–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinic P, Schlappe BA, Conlon N, Tseng J, Olvera N, Dao F, Mueller JJ, Hussein Y, Soslow RA, Levine DA. 2016. Concomitant loss of SMARCA2 and SMARCA4 expression in small cell carcinoma of the ovary, hypercalcemic type. Mod Pathol 29: 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian-Philippe M, Velasco V, Longy M, Floquet A, Arnould L, Coindre JM, Le Naoures-Méar C, Averous G, Guyon F, MacGrogan G, et al. 2015. SMARCA4 (BRG1) loss of expression is a useful marker for the diagnosis of ovarian small cell carcinoma of the hypercalcemic type (ovarian rhabdoid tumor): a comprehensive analysis of 116 rare gynecologic tumors, 9 soft tissue tumors, and 9 melanomas. Am J Surg Pathol 39: 1197–1205. [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Oliva E, Connolly LE, McBride HA, Young RH. 2004. An immunohistochemical analysis of ovarian small cell carcinoma of hypercalcemic type. Int J Gynecol Pathol 23: 330–336. [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Daya F, Ip P, Malpica A, Oliva E, Young RH. 2014. Miscellaneous tumors. In WHO classification of tumours of female reproductive organs, 4th ed. (ed. Kurman RJ, Carcangiu ML, Herrington CS, Young RH), pp. 70–71. International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- Ramos P, Karnezis AN, Craig DW, Sekulic A, Russell ML, Hendricks WP, Corneveaux JJ, Barrett MT, Shumansky K, Yang Y, et al. 2014. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet 46: 427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond VM, Gray SW, Roychowdhury S, Joffe S, Chinnaiyan AM, Parsons DW, Plon SE; Clinical Sequencing Exploratory Research Consortium Tumor Working Group. 2016. Germline findings in tumor-only sequencing: points to consider for clinicians and laboratories. J Natl Cancer Inst 108: djv351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully RE. 1979. Tumors of the ovary and maldeveloped gonads. In Atlas of tumor pathology (ed. Hartmann WH, Cowan WR), second series, fascicle 16, pp. 239–312. Armed Forces of Institute of Pathology, Washington, DC. [Google Scholar]
- Trotter KW, Archer TK. 2008. The BRG1 transcriptional coregulator. Nucl Recept Signal 6: e004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski L, Foulkes WD. 2015. In brief: picturing the complex world of chromatin remodelling families. J Pathol 237: 403–406. [DOI] [PubMed] [Google Scholar]
- Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, Grynspan D, Saloustros E, Nadaf J, Rivera B, et al. 2014. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet 46: 438–443. [DOI] [PubMed] [Google Scholar]
- Witkowski L, Goudie C, Foulkes WD, McCluggage WG. 2016. Small-cell carcinoma of the ovary of hypercalcemic type (malignant rhabdoid tumor of the ovary): a review with recent developments on pathogenesis. Surg Pathol Clin 9: 215–226. [DOI] [PubMed] [Google Scholar]
- Young RH, Oliva E, Scully RE. 1994. Small cell carcinoma of the ovary, hypercalcemic type: a clinicopathological analysis of 150 cases. Am J Surg Pathol 18: 1102–1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.