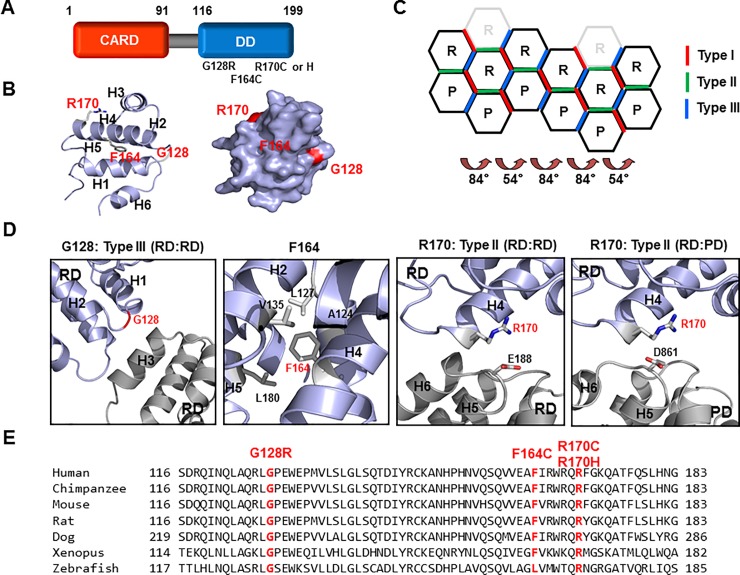
Fig 1. Locations of the point mutations in the corresponding RAIDD-TLIS variants.
A. Domain organization of RAIDD. CARD: Caspase recruiting domain, DD: Death domain. The corresponding amino acids are shown above the protein domains. Point mutations associated with TLIS are indicated under the death domain. B. Cartoon structure of RAIDD DD. The six helix bundles are named H1 to H6. The positions of TLIS-causing point mutations on RAIDD DD are indicated in red color. Right panel shows the surface of the RAIDD DD. C. Schematic planar diagram showing the formation of the RAIDD DD:PIDD DD complex. The positions of the three different types of interfaces are shown. The angles of two different types of screw rotations are shown. R and P indicate RAIDD DD and PIDD DD, respectively. D. Protein-protein interface (PPI) and the location of each mutation on RAIDD DD in the RAIDD DD:PIDD DD complex. The residues responsible for TLIS pathogenesis are shown in red. Residues that are critical for the interactions are labelled. RD and PD indicate RAIDD and PIDD, respectively. E. Cross-species alignment of the amino acid sequences of RAIDD DD. The positions of RAIDD-TLIS variants are shown in red.