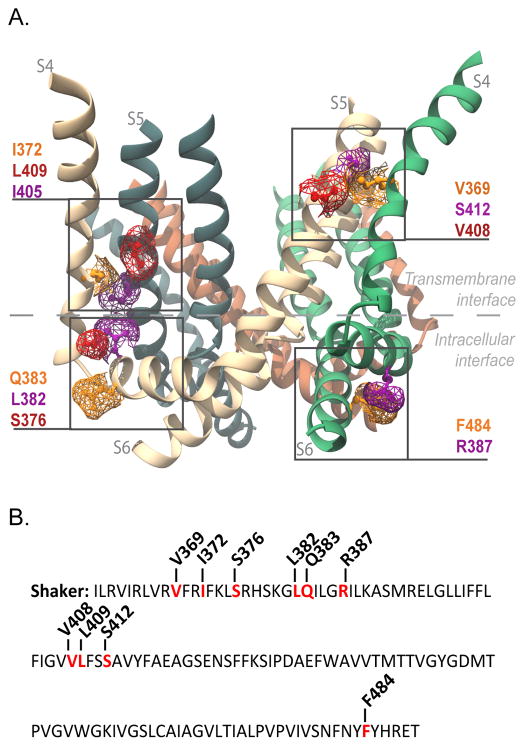
Figure 1. Interfacial regions and residues tested for electromechanical coupling.
(A) Side view of Kv1.2/2.1 chimera (PDB 2R9R). Only S4, S4–S5 linker, S5 and S6 helices are shown for clarity. Highlighted in purple, orange and red are the residues in the transmembrane gating interface of S4 and S5 of neighboring subunits (V369 with V408 and S412; I372 with I405 and L409), and those at the intracellular gating interface (S4–S5 linker (R387) with S6 (F484), and the S4 (S376) with S4–S5 linker (L382 and Q383) of the same subunit). The residue numbering corresponds to positions in the Shaker potassium channel (see Supplementary Figure 6 for alignment). (B) Shaker sequence from residue I360 to T489. Residues that were mutated to alanine are in red and the position noted.