Abstract
Visual attention is essential for visual perception. Spatial attention allows us to grant priority in processing and selectively process information at a given location. In this paper, I explain how two kinds of spatial attention: covert (allocated to the target location, without accompanying eye movements) and presaccadic (allocated to the location of the upcoming saccade’s target) affect performance and alter appearance. First, I highlight some behavioral and neuroimaging research on covert attention, which alters performance and appearance in many basic visual tasks. Second, I review studies showing that presaccadic attention improves performance and alters appearance at the saccade target location. Further, these modulations change the processing of feature information automatically, even when it is detrimental to the task at hand. We propose that saccade preparation may support transaccadic integration. Systematically investigating the common and differential characteristics of covert attention and presaccadic attention will continue to further our understanding of the pervasive selective processing of information, which enables us to make sense of our complex visual world.
1. INTRODUCTION
Each time we open our eyes we are confronted with an overwhelming amount of information. Yet, we seemingly understand our visual world effortlessly. To make sense of a scene, we need to detect, localize, and identify relevant information. Owing to the high-energy cost of neuronal activity, only a very small fraction of cortical neurons can be active (above base level) at a given time, and we cannot process all this information equally well. Knowledge and assumptions about the world, the behavioral state of the organism, and the (sudden) appearance of possibly relevant information in the environment, facilitate the processing of sensory input. By focusing on a certain location or aspect of the visual scene, attention allows us to selectively process information, prioritizing some aspects of information while ignoring others.
Attention lies at the crossroads between perception and cognition. Significant advances in visual attention have been facilitated by fruitful cross talk among scientist working at different levels of analyses, using different techniques, such as psychophysics, neurophysiology, neuroimaging, and computational modeling. Attention can be allocated by moving one’s eyes toward a location (overt attention) or by attending to an area in the periphery without actually directing one’s gaze toward it (covert attention).
Because spatial resolution, our ability to discriminate two nearby points in space, decreases pronouncedly with retinal eccentricity, we humans move our eyes thousands of times per day, to foveate relevant information and to increase spatial resolution. The deployment of covert attention aids us in monitoring the environment and can guide eye movements (overt attention) to locations of the visual field where salient and/or relevant information is likely to be. We also deploy covert attention routinely in many everyday situations, such as searching for objects, crossing the street, driving, dancing and playing sports. Furthermore, covert attention plays an important role in social situations, when moving the eyes provides a cue to intentions that we wish to conceal. For instance, when you see someone at a conference and you have forgotten her name, you might want to look at her nametag, but that would reveal that you had forgotten her name. Covert attention enhances visual processing allowing us to keep our gaze at the other person’s face while reading the nametag. Covert attention improves various basic aspects of visual processing, such as contrast sensitivity, spatial resolution and speed of information accrual (for reviews see, Anton-Erxleben & Carrasco 2013; Carrasco 2006, 2011, 2014; Carrasco & Barbot 2015; Carrasco & Yeshurun 2009).
There is an interaction between covert and overt attention. Sometimes, we attend covertly to a location without programming an eye movement. Other times, we shift covert spatial attention first and then make an eye movement to further inspect the object that caught our interest. The current view is that a saccadic eye movement often but not necessarily follows a covert shift of attention, but that planning a saccadic eye movement is preceded by a shift of attention, a phenomenon termed, presaccadic attention.
Selective attention arises from the brain’s limited capacity to process information. There is a fixed amount of overall energy available to the brain and the high bioenergetic cost of the neuronal activity involved in cortical computation. For those reasons, our brain uses efficient representational codes that rely on a sparse collection of active neurons, and flexibly allocates metabolic resources according to task demands. The energy limitations allow only a small fraction of the brain machinery to be engaged concurrently, and provide a neurophysiological basis for selective attention (Lennie 2003; Carrasco 2011).
2. COVERT ATTENTION
Even with very simple displays, attention is involved in distributing resources across the visual field. There are processing trade-offs for simple, non-cluttered displays, in which only two stimuli are competing for processing; the benefit at the attended location for contrast sensitivity and acuity has a concomitant cost at the unattended location (Barbot, Landy & Carrasco 2011, 2012; Herrmann, Montaser-Kouhsari, Carrasco & Heeger 2010; Giordano, McElree & Carrasco 2009; Lu & Dosher 1998; Luck, Hillyard, Mouloua, Woldorff, Clark & Hawkins 1994; Montagna, Pestilli & Carrasco 2009; Pestilli & Carrasco 2005; Pestilli, Viera & Carrasco 2007; Yeshurun & Rashal 2010). These findings suggest that trade-offs are a characteristic of attentional allocation, which has a general effect across different visual tasks.
There are two types of covert attention that deal with facilitation and selection of information: endogenous and exogenous. The former is a voluntary system that corresponds to our ability to willfully monitor information at a given location; the latter is an involuntary system that corresponds to an automatic orienting response to a location where sudden stimulation has occurred. These two types of attention also have different temporal dynamics. Endogenous attention takes about 300 ms to be deployed and has a sustained response whereas exogenous attention has a transient response, it peaks by 100 ms and rises and decays quickly (e.g., Muller & Rabbitt 1989; Nakayama & Mackeben 1989; Remington, Johnston & Yantis 1992; Liu, Stevens & Carrasco 2007). The effects of attention on performance scale with cue validity, but those of exogenous attention do not (Sperling & Melchner 1978; Kinchla 1980; Mangun & Hillyard 1990; Yantis & Jonides 1996; Giordano et al. 2009). Exogenous attention affects performance even when cues are known to be uninformative (Barbot et al. 2011; Herrmann et al. 2010; Montagna et al. 2009; Pestilli & Carrasco 2005; Pestilli et al. 2007; Yeshurun & Rashal 2010) and even when they impair performance (Bocanegra & Zeelenberg 2011; Carrasco, Loula & Ho 2006; Hein, Rolke & Ulrich 2006; Talgar & Carrasco 2002; Yeshurun & Carrasco 1998, 2000, 2008; Yeshurun & Levy 2003). These findings suggest that exogenous attention may be phylogenetically older than endogenous attention, allowing us to respond automatically and quickly to stimuli that may provide behaviorally relevant information.
Here I review studies that deal with the effects of covert attention—endogenous or exogenous—on perceptual performance of detection and discrimination tasks, as well as by altering the subjective appearance of a stimulus or object. Covert attention has been studied sometimes in the parafovea (1°–5°) and mostly in the perifovea (5°–10°) and periphery (>10° of eccentricity). I concentrate on studies using an orientation discrimination task. This dimension has been well characterized both psychophysically and neurophysiologically and a link between such findings has been well established (e.g., De Valois & De Valois 1988; Graham 1989; Ringach, Hawken & Shapley 1997). In addition, orientation discrimination is used to assess the effect of attention on contrast sensitivity because performance on this task improves with increasing contrast (Nachmias 1967; Skottun, Bradley, Sclar, Ohzawa & Freeman 1987), and because fMRI response increases monotonically with stimulus contrast (Boynton, Demb, Glover & Heeger 1999).
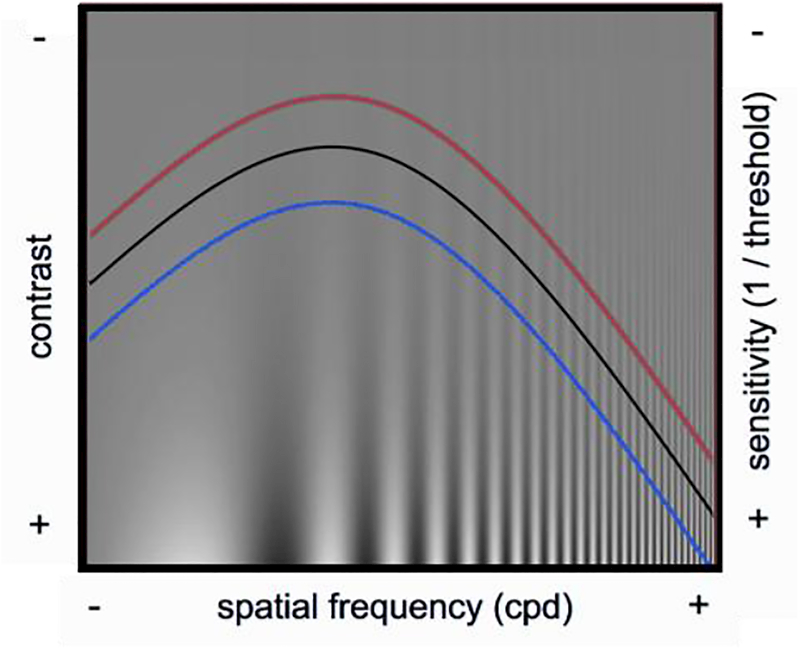
The contrast sensitivity function (CSF, Figure 1) depicts observers contrast sensitivity as a function of spatial frequency (sinusoidal changes of dark and bright light). This contrast sensitivity function has been considered to be the window of visibility. We see what is below the black curve, but not what is above the black curve. This CSF changes at different retinal locations; our sensitivity decreases with stimulus eccentricity. Many years ago, we set out to investigate whether exogenous attention would increase sensitivity (hypothetical red curve) at the attended location and would decrease sensitivity at the unattended location (hypothetical blue curve).
Figure 1.
Contrast sensitivity function. The hypothetical curves illustrate contrast sensitivity under neutral condition (black), at the attended location (red) and unattended locations (blue).
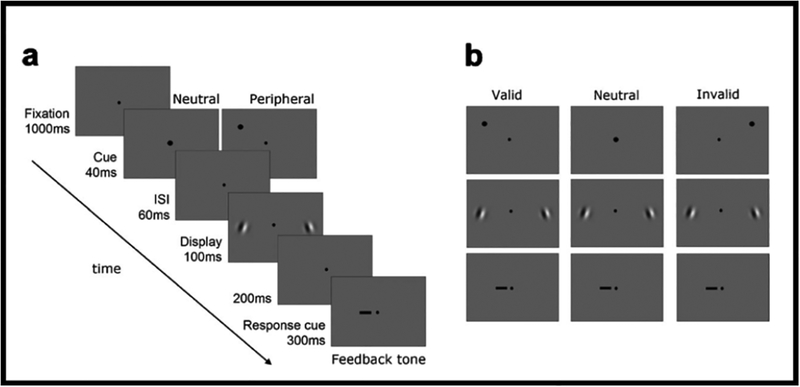
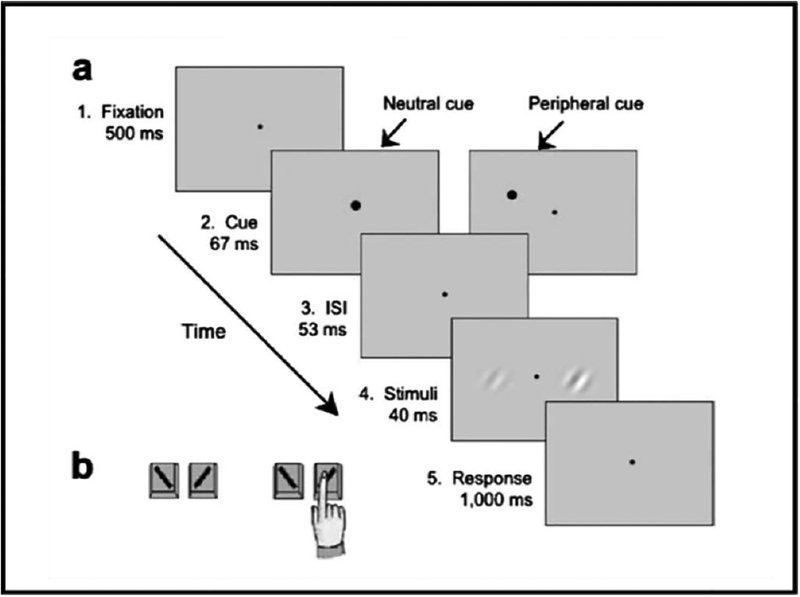
To isolate the effect of covert attention, it is important to manipulate attention while keeping stimulus and task constant with the same observers. Figure 2a shows a trial sequence, in which exogenous attention is manipulated. Following a fixation point, a cue appeared either above one of the two Gabor locations (peripheral cue) or at fixation (neutral cue). After an ISI, two Gabors were simultaneously presented (randomly oriented to the left or to the right) on the horizontal meridian. Then a response cue appeared at fixation to indicate the target Gabor for which the observer had to report the orientation. Figure 2b illustrated the three types of trials. Observers were told that the cue was uninformative: On one third of the trials, the response cue pointed to the precued Gabor (valid cue). On another third of the trials, it pointed to the Gabor that was not precued (invalid cue). In the remaining trials, the precue was presented in the center of the screen and the response cue was equally likely to indicate the Gabor to the right or to the left of fixation (neutral cue).
Figure 2.
Protocol to assess effects of exogenous attention at cued and uncued locations. (a) A trial sequence. (b) Examples of types of trials (Pestilli & Carrasco, 2005, Fig. 1).
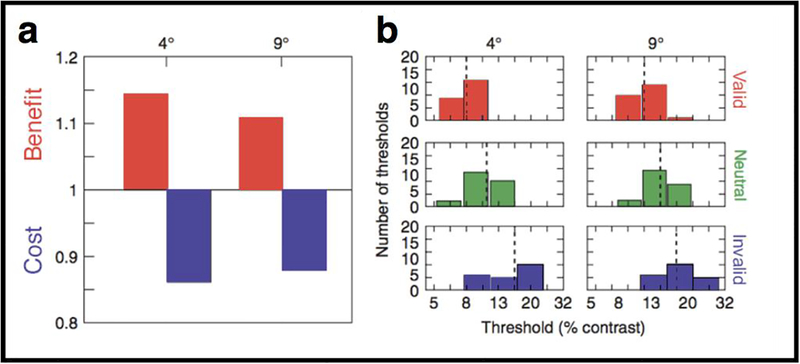
Using this protocol, we showed that exogenous attention increases contrast sensitivity at the cued location and decreases it at the uncued location. Figure 3a depicts the ratios of the medians of the sensitivity (1/median threshold) for one observer and indicates a benefit of allocating attention to the target location (valid:neutral ratio: sensitivity in the valid condition is higher than sensitivity in the neutral condition). A ratio of less than one indicates a cost of allocating attention to the nontarget location (invalid:neutral ratio: sensitivity in the invalid condition is lower than sensitivity in the neutral condition). Figure 3b contains six histograms representing the thresholds obtained for the same observer in each cue condition. Red histograms represent the threshold obtained for the valid condition; green histograms represent the neutral cue condition; blue histograms represent the invalid cue condition (Adapted from Pestilli & Carrasco 2005, Fig. 2.)
Figure 3.
Effects of exogenous attention at cued and uncued locations. (a) The left panel depicts the ratios of the medians of the sensitivity (1/median threshold) for one observer for 4 deg and 9 deg eccentricity. (b) The right panel contains six histograms representing the thresholds obtained for the same observer in each cue condition at 4 deg and 9 deg eccentricity. Black vertical dotted lines indicate the median values. (Adapted from Pestilli & Carrasco, 2005, Fig. 2.)
In a series of studies we have shown that compared to a neutral condition, exogenous and endogenous attention enhance contrast sensitivity for a wide range of spatial frequencies at the attended location and decrease sensitivity at unattended locations across the visual field, at different eccentricities and isoeccentric (polar angle) locations (Carrasco, Penpeci-Talgar & Eckstein 2000; Carrasco, Giordano & McElree 2004, 2006; Cameron, Tai & Carrasco 2002; Giordano et al. 2009; Herrmann et al. 2010; Ling & Carrasco 2006; Pestilli & Carrasco 2005; Pestilli et al. 2007; Pestilli, Ling & Carrasco 2009).
We, and others had found that with a constant stimulus size, exogenous attention alters performance via a response gain change, increased performance by upward scaling of the psychometric function, whereas endogenous attention does so via a contrast gain change, increased performance resulting from multiplying the stimulus contrast by a scale factor (Pestilli & Carrasco 2005; Pestilli et al. 2007; Ling & Carrasco 2006; Huang & Dobkins 2005; Di Russo, Spinelli & Morrone 2001).
The Normalization Model of Attention by Reynolds and Heeger (2009) was proposed to reconcile previous, seemingly contradictory findings on the effects of visual attention and to offer a computational framework to simulate new research questions. According to the model, spatial attention can be characterized with an attention field selective for a given spatial location. The stimulus drive is multiplied with the attention field and then normalized, such that the extent of the stimulus and the relative extent of the attention field can shift the balance between excitation and suppression. Thus, the model can exhibit different effects of attentional modulation, such as response gain, changes that increase firing rates by a multiplicative scale factor without changing the shape or width of neuronal tuning (e.g., Treue & Maunsell 1999); contrast gain changes that increase responses, multiplying the stimulus contrast by a scale factor (e.g., Reynolds, Pasternak & Desimone 2000); or a combination of both response gain and contrast gain changes (Williford & Maunsell 2006).
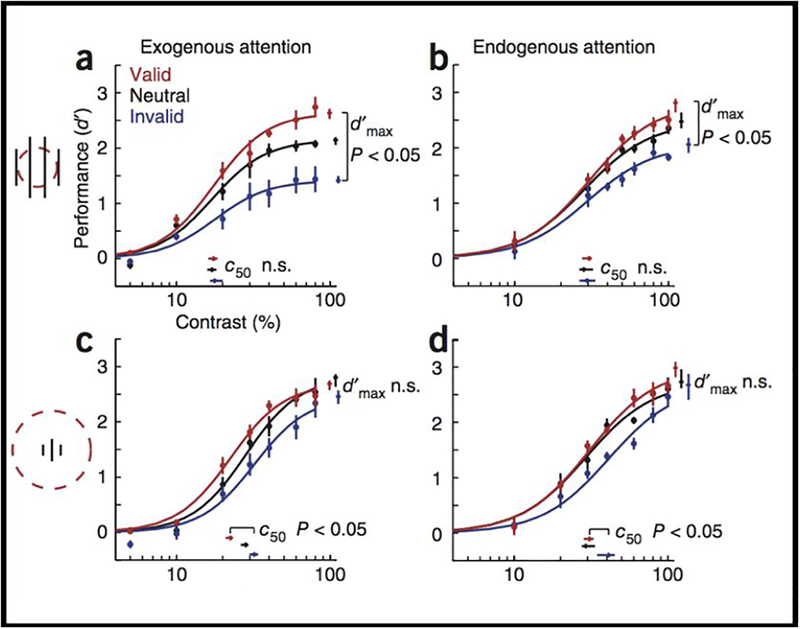
We confirmed a key model perfection, that the effect of attention can systematically shift from a change in contrast gain to response gain with larger stimuli and a smaller attention field. Figure 4 shows plots of psychometric functions for each attentional condition (valid, neutral and invalid precues) and parameter estimates (c50, contrast yielding half-maximum performance; dmax, asymptotic performance at high contrast). When the stimuli are large and the size of the window is small, both exogenous and endogenous attention yield response gain (Figure 4a, b). In contrast, when the stimuli are small and the size of the window is large, both exogenous and endogenous attention yield contrast gain (Figure 4c, d). An fMRI experiment confirmed that the attention field was larger with spatial uncertainty than without it (Herrmann et al. 2010).
Figure 4.
Effects of exogenous and endogenous attention on performance (d’) as a function of contrast. (a,b) Large stimulus with small attention field. (c,d) Small stimulus with large attention field. Each data point represents the mean across observers. Error bars on data points are s.e.m. Error bars on parameter estimates are confidence intervals obtained by bootstrapping. (Reprinted from Herrmann, Montaser-Koushari, Carrasco & Heeger, 2010, Fig. 3).
Analyzing previous findings in the literature, we concluded that response gain and contrast gain changes in behavioral studies could have been elicited by the placement of cues that may have encouraged differences in attention field size and differences in stimulus size. For instance, for endogenous attention, response gain changes were reported with larger stimuli (Morrone, Denti & Spinelli 2002, 2004), a combination of contrast and response gain changes was observed with intermediate stimulus sizes (Huang & Dobkins 2005) and contrast gain changes were reported with smaller stimuli (Ling & Carrasco 2006).
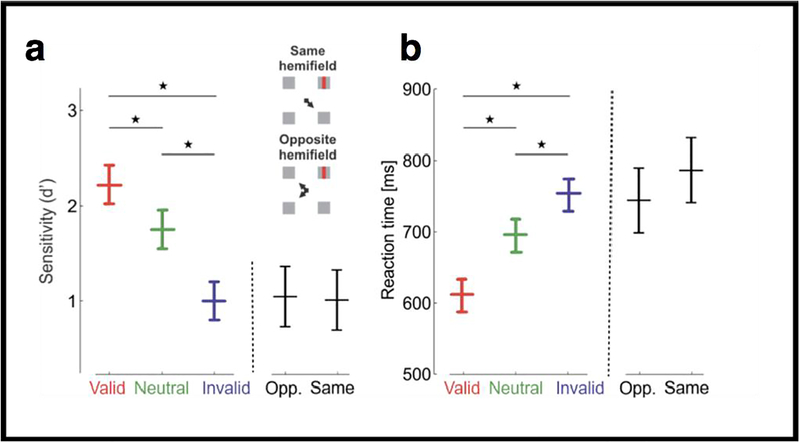
Covert attention has been studied sometimes in the parafovea (1°–5°) and mostly in the perifovea (5°–10°) and periphery (>10° of eccentricity); that is, far outside the foveola, the high-acuity region of the retina at the center of gaze. The foveola is the region of the fovea that is rod- and capillary-free where cones are most densely packed, an area that covers only ~1° of visual angle, or about the size of the index fingernail at arm’s length. The wide-held assumption has been that there is no need for selective processing in this region, as we are already directly looking at the stimuli. Furthermore, until recently, there were no methods to directly investigate this issue. In a recent study we used two methods for precisely controlling retinal stimulation, namely retinal stabilization and a gaze-contingent calibration procedure (Poletti, Listorti & Rucci 2013). We found that covert attention flexibly improves (Figure 5a) and speeds up (Figure 5b) both detection and discrimination at the loci at only a fraction of a degree (14 arcmin) apart within the foveola. Moreover, these benefits have concurrent costs at other locations, and that the magnitude of such effect is the same regardless of whether the invalid cue pointed to a location in the same or in the opposite hemifield. These findings reveal a surprisingly precise control of covert attention and its involvement in fine spatial vision. Furthermore, they show that covert attention is a mechanism that exerts its action across the entire visual field, even at locaton where we are already looking, the region of highest acuity (Poletti, Rucci & Carrasco 2017).
Figure 5.
Effects of covert attention in the foveola. Experimental results, measured as (a) accuracy (d`) and (b) reaction times, for different trial types across observers. Differences between valid and invalid trials were statistically significant for all individuals. Accuracy and reaction times are also shown separately for the invalid trials in which the cue and the target were presented in the same and opposite hemifield, respectively. Error bars are 95% CI. *P < 0.05 (Reprinted from Poletti, Rucci & Carrasco, 2017, Fig. 3).
From psychophysical and neurophysiological evidence indicating that covert attention increases contrast sensitivity, one might infer that attention changes contrast appearance. Whether attention can actually affect the perceived intensity of a stimulus has been a matter of debate dating back to the founding fathers of experimental psychology and psychophysics—Helmholtz, James, and Fechner. Indeed, psychologists, physiologists, and philosophers have debated the phenomenology of attention since the late 19th century. Does attention alter our subjective experience of the visual world? Can attention make a visual pattern seem more detailed, or a color more vivid? Studies on the phenomenological correlates of attention, which are relevant to the topic of subjective experience and awareness, show that attention alters appearance of basic spatial (e.g., contrast, spatial resolution, color saturation, object size) and temporal (e.g., flicker rate, motion coherence, speed) visual dimensions (reviews by Carrasco 2011, 2014; Anton-Erxleben & Carrasco 2013).
Whether and how attention affects appearance has been systematically investigated only recently. This may be due to the difficulty in objectively testing and quantifying the subjective experience of perceived stimuli and changes in such experience with attention. A psychophysical paradigm that assesses the phenomenological correlate of attention, by manipulating exogenous attention via uninformative spatial cues, makes it possible to study subjective experience and visual awareness more objectively and rigorously (Luck 2004; Treue 2004).
This psychophysical paradigm quantifies the observer’s subjective perception using a task contingent upon a comparative judgment between two stimuli on a particular feature (Carrasco, Ling & Read 2004; Figure 6). Observers are shown two stimuli and asked to ‘report property x of the stimulus that is greater/lesser in property y’. That is, the perceived relative values of property y—the primary interest of the experiment (e.g. contrast)—is an indicator of which stimulus to report on property x (e.g. orientation). The critical manipulation is that observers are not asked to directly rate their subjective experience on property y, but to make a decision about stimulus property x. By using a 2×2 alternative forced-choice task, we asked observers to indicate the orientation (left vs. right) for the stimulus that appeared higher in contrast. This paradigm allows us to simultaneously measure the effect of attention on appearance and performance.
Figure 6.
Protocol to assess attention effects on appearance. (a) Sequence of events in a single trial. Each trial began with a fixation point followed by a brief neutral or peripheral cue. The peripheral cue had equal probability of appearing on the left- or right-hand side, and was not predictive of the stimulus contrast or orientation. The timing of this sequence maximized the effect of transient exogenous attention and precluded eye movements. (b) Task. Observers performed a 2X2 forced choice (2X2 AFC) task: they were asked to indicate the orientation (left vs. right) for the stimulus that appeared higher in contrast. In this trial, they would report the orientation for the stimulus on the right. (Reprinted from Carrasco, Ling & Read 2004, Fig. 1.)
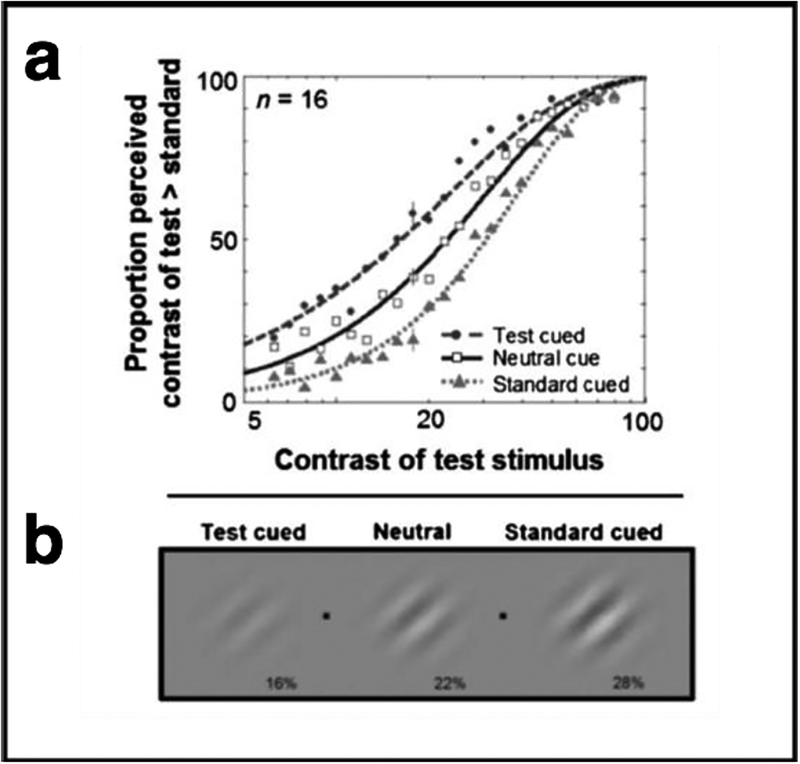
Exogenous attention significantly increased perceived contrast. Figure 7a shows the percentage of responses in which observers reported the contrast of the test patch as higher than the standard, plotted as a function of the physical contrast of the test patch. Data are shown for the neutral and peripheral conditions (test cued and standard cued). The standard was 22% contrast and that is the contrast at which the test and standard stimuli attained subjective equality (50%). When a Gabor stimulus was peripherally cued, the point of subjective equality (PSE) was shifted – the apparent contrast of the stimulus for which exogenous attention had been drawn to was higher than when attention was not drawn there. That is to say, when observers attend to a stimulus, they perceive it to be of significantly higher contrast than when they perceive the same stimulus without attention (Carrasco et al. 2004). This effect has been obtained for a wide range of test stimulus contrasts (Cutrone, Heeger & Carrasco 2014). Figure 7b illustrates the effect of covert attention on apparent contrast. If you were looking at one of the two fixation points (black dots), and the grating to the left of that fixation point was cued, the stimuli at both sides of fixation would appear to have the same contrast. This example illustrates that a cued 16% contrast grating appears as if it were 22% contrast, and a cued 22% contrast grating appears as if it were 28% contrast.
Figure 7.
Attention alters appearance. (a) Appearance psychometric function. Percentage of responses in which observers reported the contrast of the test patch as higher than the standard, plotted as a function of the physical contrast of the test patch. Data are shown for the neutral and peripheral conditions (test cued and standard cued). (b) Effect of covert attention on apparent contrast illustrating the point of subjective equality for the different cueing conditions (Adapted from Carrasco et al., 2004, Figs. 4 and 5).
Multiple control experiments have ruled out alternative accounts of these findings based on cue bias or response bias with regard to perceived contrast. For instance, the appearance effect disappears when instead of precueing the display, we use postcues, or when instead of having the optimal interval between cue onset and stimuli for exogenous attention, we have a longer interval at which the transient effect of exogenous attention is no longer effective (Anton-Erxleben, Abrams & Carrasco 2010, 2011; Carrasco, Fuller & Ling 2008; Carrasco et al. 2004; Fuller, Rodriguez & Carrasco 2008; Fuller, Park & Carrasco 2009; Ling & Carrasco 2007). The appearance paradigm has been adapted to investigate the effect of endogenous voluntary attention, which also has been shown to increase perceived contrast (Liu, Abrams & Carrasco 2009). As is the case for exogenous attention, a number of control experiments have ruled out alternative explanations for endogenous attention.
In short, covert attention alters performance and appearance. Both exogenous and endogenous attention increase contrast sensitivity at the attended location while concurrently decreasing it at the unattended locations. Likewise, perceived contrast increases at the attended location while concurrently decreasing it at unattended locations.
Presaccadic Attention
Large, rapid eye movements, called saccades, largely shape the way we see. Humans and primates constantly make saccades, so that behaviorally relevant objects can be positioned at the fovea where acuity is greatest. Saccades swiftly point the fovea to locations of interest, ensuring high-acuity vision for the scene’s relevant information. Saccades induce rapid changes of the retinal images, and how the visual system maintains a stable percept across eye movements has intrigued neuroscientists for decades. The enhanced processing of the targets of saccades starts even before the eyes begin to move (Kowler, Anderson, Dosher & Blaser 1995; Deubel & Schneider 1996). Observers show pronounced improvements in performance in visual discrimination tasks, starting 100 ms before a saccade (Castet, Jeanjean, Montagnini, Laugier & Masson 2006; Montagnini & Castet 2007; Deubel 2008; Li, Barbot & Carrasco 2016; Rolfs, Jonikaitis, Deubel & Cavanagh 2011;, Rolfs & Carrasco 2012). This effect–presaccadic attention–is accompanied by enhanced neural responses in frontoparietal and sensory cortices, considered to prioritize the saccade target and underlie the improved performance (Bisley & Goldberg 2010; Moore & Zirnsak 2017).
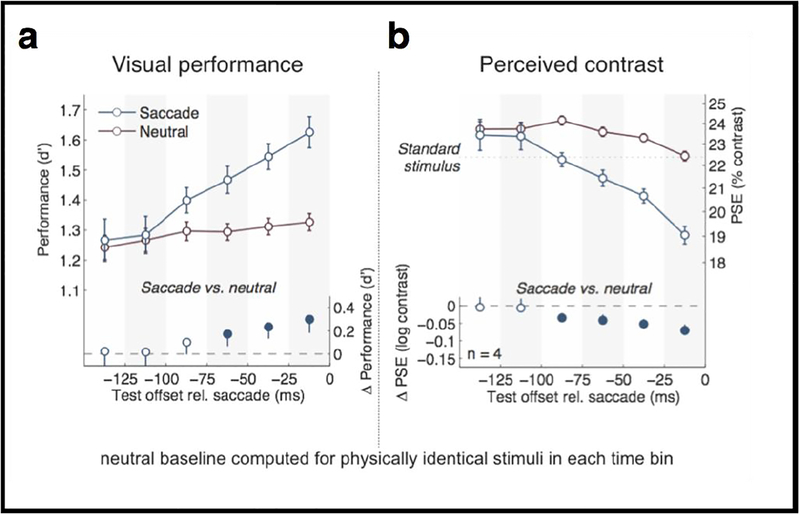
We implemented a novel behavioral task to simultaneously assess effects of saccade preparation on contrast sensitivity and perceived contrast (Rolfs & Carrasco 2012), inspired by our psychophysical paradigm developed to quantify subjective, phenomenological correlates of covert attention (Carrasco et al. 2004; Liu et al. 2009). We asked observers to simultaneously judge both the orientation and the contrast of a test stimulus presented at the target of a cued saccadic eye movement relative to a standard stimulus shown during prior fixation. Figure 8 plots performance for the orientation discrimination task (Figure 8a) and perceived contrast (Figure 8b) while observers are preparing to saccade, as a function of saccade onset. We found simultaneous progressive enhancement in both orientation discrimination performance and perceived contrast as time approached saccade onset. These effects were robust as early as 60 ms after the eye movement was cued, much faster than the voluntary deployment of covert attention (without eye movements), which takes 300 ms. Our results link the dynamics of saccade preparation, visual performance, and subjective experience and show that upcoming eye movements alter visual processing by increasing the signal strength (Rolfs & Carrasco 2012).
Figure 8.
Result of presaccadic attention. Dynamic perceptual changes before saccades. Average changes in (a) orientation discrimination performance, and (b) perceived contrast, as a function of stimulus offset relative to saccade onset. (Adapted from Rolfs & Carrasco, 2012, Figure 3).
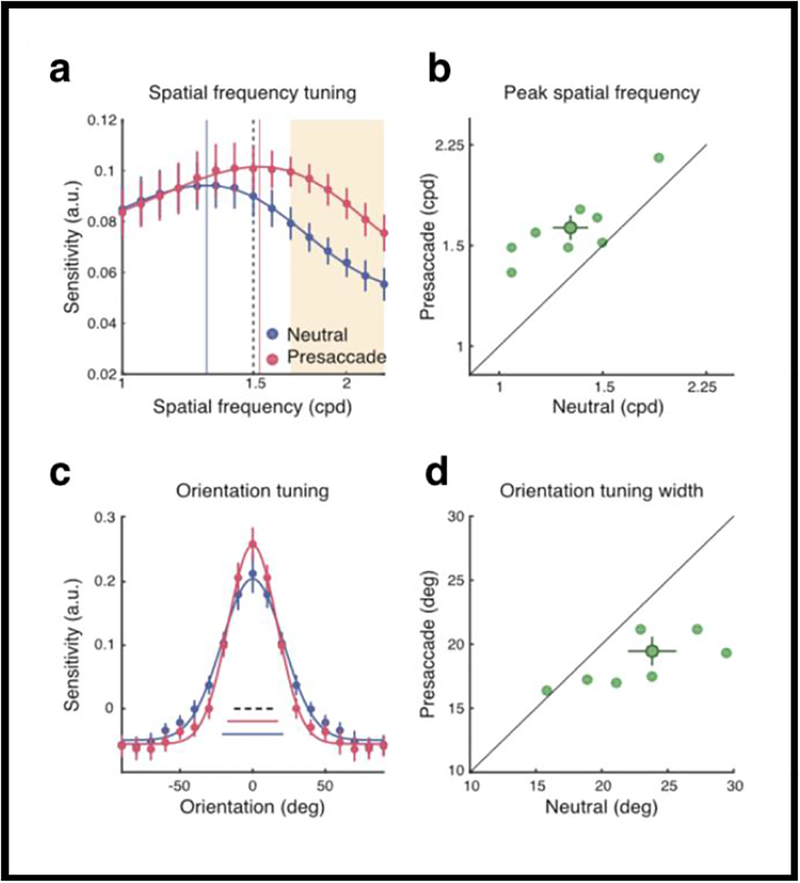
To investigate whether and how presaccadic modulations change the processing of feature information and thus modulate visual representations, we used reverse correlation, a method which enables experimenters to quantify the contribution of different features to performance in perceptual tasks (e.g., Ahumada 2002; Wyart, Nobre & Summerfield 2012). Specifically, reverse correlation enabled us to assess how saccade preparation affects the way the visual system assigns weights to different spatial frequency (SF) and orientation contents to reach a perceptual judgment. Observers detected a vertical target embedded in random noise presented at 10 degrees left or right of fixation. Figure 9a shows that visual sensitivity was enhanced for higher SFs than that of the stimulus. Figure 9b shows this was the case for all observers (except one whose data point is on the diagonal line, indicating that this observer’s sensitivity was the same for both conditions). Figure 9c indicates that orientation tuning was narrower for the presaccadic than the neutral condition. Figure 9d shows this was the case for all observers (except one whose data point is on the diagonal line, indicating that this observer’s orientation tuning was the same for both conditions). To establish whether this effect could be due to covert attention rather than to saccade planning, we conducted the same experiment but instructed observers not to saccade (and monitored their eye movements). There was no significant difference in performance between the neutral and the covert attention conditions. Our findings demonstrated a strong coupling between visual representation and motor output: saccade preparation not only enhanced visual sensitivity but reshaped sensory tuning by enhancing the gain of high SF information and by narrowing the width of orientation tuning. These modulations are automatic processes driven by extra-retinal preparatory signals related to eye movements and not due to mere covert attention.
Figure 9.
Saccade Preparation Reshapes Sensory Tuning (a) Group-averaged SF tuning functions. Error bars denote ±1 SEM. The vertical lines represent the peak frequency. The dashed line represents the target Gabor. The orange shaded area represents the cluster of SF channels that showed a significant difference between the two conditions. (b) The peak frequency of the presaccadic interval was plotted against the peak frequency of the neutral condition. The dark green data point and error bars represent group mean and ±1 SEM. The light data points represent individual parameter fits. (c) Group-averaged orientation tuning functions. Error bars denote ±1 SEM. The three horizontal bars at the bottom denote the fitted tuning widths (±1 s; the dashed line denotes the width of the target Gabor). (d) The presaccadic tuning width was plotted against the neutral tuning width. The dark data point and error bars represent group mean and ±1 SEM. The light data points represent individual parameter fits. (Reprinted from Li, Barbot & Carrasco, Fig. 4)
We were particularly interested in understanding the nature of the heightened sensitivity for high SFs that we found. In particular, we investigated whether this heightened sensitivity occurs only when it is beneficial for performance for the task at hand, or whether it occurs as an automatic functional heuristic. Were automatic shiftthe case, presaccadic attention would hamper performance in situations in which increased sensitivity for high SF would be detrimental for the task at hand. To disentangle these two possibilities, we presented a target embedded in noise, which could be of lower, the same or higher SF than the target. In this design, if presaccadic attention automatically increases sensitivity for high SFs, performance would be impaired for the target masked by the high SF mask. Alternatively, if presaccadic attention only enhanced high SFs when it is beneficial for the task at hand, we would expect no impairment in performance.
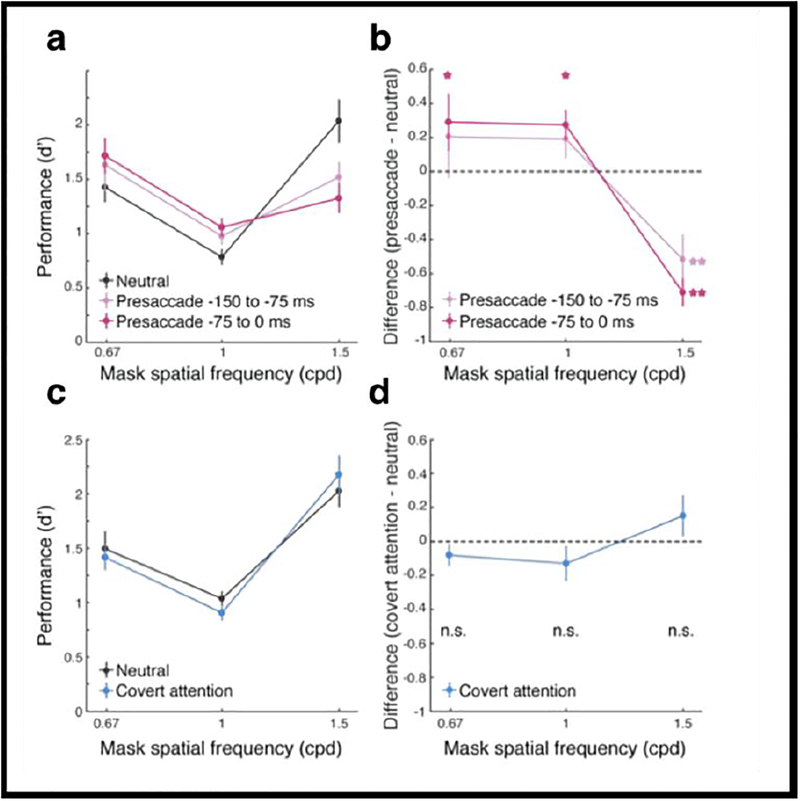
Figure 10a shows performance for three masks of different SF content. First, note that consistent with previous studies, performance was lower when the mask had the same SF content as the target than when it had lower or higher SF content than the target. Moreover, in the presaccadic condition, performance was improved when the mask’s SF was of lower or the same SF as the target, but impaired performance when the mask had higher SF content than the target. This pattern of responses was more pronounced as the stimulus was presented more proximal to saccade onset. Figure 10b plots the performance difference between the two conditions, presaccadic and neutral, and indicates the significant differences. To establish whether this effect could be due to covert attention rather than to saccade planning, we conducted the same experiment but instructed observers not to saccade (and monitored their eye movements). Figures 10c and d indicate that there was no significant difference in performance between the neutral and the covert attention condition for any of the three masks, thus ruling out the role of covert attention for our findings. In all, these results show that the enhanced sensitivity to high SFs occurs automatically even when it can be detrimental for the task at hand. This automatic enhancement can facilitate transaccadic integration, enabling the presaccadic representation of the target to be more similar to its post-saccadic (foveal) image. This is, because the fovea’s receptors are tuned for higher frequencies and narrower orientation tuning than the receptors at the peripheral location the target occupied while observers planned the saccade.
Fig 10.
(a) Performance (d’) as a function of mask SFs. Error bars represent standard deviation of the bootstrapped distribution. (b) Difference of d’ between the saccade and neutral conditions. Saccade preparation improved performance for the low- and same-SF masks but decreased it for the high-SF mask. Error bars represent standard deviation of the bootstrapped distribution. *=p<.05; **=p<.005. (c–d) Control experiment. Corresponding to panels (a–b) for performance in the neutral and covert-attention conditions. n.s.=no significant difference. (Li, Pan & Carrasco, 2018).
To conclude, we have shown that presaccadic attention improves performance and enhances perceived contrast of the saccade target, right before we move our eyes while planning a saccadic eye movement. This effect seems to be mediated by increased contrast sensitivity, heightened sensitivity for high SFs and orientation tuning. Presaccadic attention not only prioritizes the saccade target but also automatically modifies its featural representation. It can either improve or impair performance depending on the spatial frequency content of the visual input. Human observers can maintain a stable percept of a saccade target by integrating its presaccadic (in the periphery) and postsaccadic (at the fovea) representations of both SF and orientation. The tuning modulations we found suggest that saccade preparation modifies the representation of the saccade target to be more fovea-like—i.e., higher resolution and finer orientation tuning—just before saccade onset.
3. CONCLUSION
In this paper, I have highlighted studies showing that both covert attention and presaccadic attention improve performance in orientation discrimination tasks, which are mediated by contrast sensitivity. Moreover, in addition to improving performance, both of them also alter appearance, by enhancing perceived contrast. However, the temporal dynamics with which they exert their effects differ; the voluntary deployment of presaccadic attention exerts its effects faster than that of covert attention. Furthermore, some of the changes in the featural representation brought about by these two types of spatial attention are common (e.g. gain), but other changes differ (e.g. presaccadic attention yields narrower orientation tuning but covert attention does not).
In a number of studies, we have shown that the effects of covert attention described here are present with a number of special populations, and to the same extent as with neurotypicals. For instance, both endogenous and exogenous attention improve performance in orientation discrimination tasks in people with ASD (autism spectrum disorder, Grubb, Behrmann, Egan, Minshew, Carrasco & Heeger 2013a, 2013b), people with ADHD (Roberts, Ashinoff, Castellanos & Carrasco 2017) and people with amblyopia (Roberts, Cymerman, Smith R, Kiorpes & Carrasco 2016). We plan to test whether presaccadic attention also exerts the same effects with these special populations as it does with neurotypical people.
In a line of research in my lab, we continue to systematically investigate the common and differential characteristics of covert attention and presaccadic attention. This research will continue to further our understanding of the pervasive selective processing of information, which enables us to make sense of our complex visual world.
References
- Ahumada AJ (2002). “Classification image weights and internal noise level estimation.” Journal of Vision 2(1): 121–131. [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K, Abrams J and Carrasco M (2010). “Evaluating comparative and equality judgments in contrast perception: attention alters appearance.” Journal of Vision 10(11): 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K, Abrams J and Carrasco M (2011). “Equality judgments cannot distinguish between attention effects on appearance and criterion: a reply to Schneider (2011).” Journal of Vision 11(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton-Erxleben K and Carrasco M (2013). “Attentional enhancement of spatial resolution: linking behavioural and neurophysiological evidence.” Nature Review Neuroscience 14(3): 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Landy MS and Carrasco M (2011). “Exogenous attention enhances 2nd-order contrast sensitivity.” Vision Research 51: 1086–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbot A, Landy MS and Carrasco M (2012). “Differential effects of exogenous and endogenous attention on second-order texture contrast sensitivity.” Journal of Vision 12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW and Goldberg ME (2010). “Attention, intention, and priority in the parietal lobe.” Annual Review of Neuroscience 33: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra BR and Zeelenberg R (2011). “Emotion-induced trade-offs in spatiotemporal vision.” Journal of Experimental Psycholology: General 140(2): 272–282. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH and Heeger DJ (1999). “Neuronal basis of contrast discrimination.” Vision Research 39(2): 257–269. [DOI] [PubMed] [Google Scholar]
- Cameron EL, Tai JC and Carrasco M (2002). “Covert attention affects the psychometric function of contrast sensitivity.” Vision Research 42(8): 949–967. [DOI] [PubMed] [Google Scholar]
- Carrasco M (2006). “Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies.” Progress in Brain Research 154: 33–70. [DOI] [PubMed] [Google Scholar]
- Carrasco M (2011). “Visual attention: the past 25 years.” Vision Research 51(13): 1484–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M (2014). Spatial Attention: Perceptual modulation The Oxford Handbook of Attention. Kastner S and Nobre AC, Oxford University Press: 183–230. [Google Scholar]
- Carrasco M and Barbot A (2015). “How Attention Affects Spatial Resolution.” Cold Spring Harbor Symposia on Quantitative Biology. 79: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Fuller S and Ling S (2008). “Transient attention does increase perceived contrast of suprathreshold stimuli: A reply to Prinzmetal, Long and Leonhardt.” Perception & Psychophysics 70(7): 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM and McElree B (2004). “Temporal performance fields: visual and attentional factors.” Vision Research 44(12): 1351–1365. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Giordano AM and McElree B (2006). “Attention speeds processing across eccentricity: feature and conjunction searches.” Vision Research 46(13): 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S and Read S (2004). “Attention alters appearance.” Nature Neuroscience 7(3): 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Loula F and Ho YX (2006). “How attention enhances spatial resolution: evidence from selective adaptation to spatial frequency.” Perception & Psychophysics 68(6): 1004–1012. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C and Eckstein M (2000). “Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement.” Vision Research 40(10–12): 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M and Yeshurun Y (2009). “Covert attention effects on spatial resolution.” Prog Brain Res 176: 65–86. [DOI] [PubMed] [Google Scholar]
- Castet E, Jeanjean S, Montagnini A, Laugier D and Masson G (2006). “Dynamics of attentional deployment during saccadic programming.” Journal of Vision. 6: 196–212. [DOI] [PubMed] [Google Scholar]
- Cutrone EK, Heeger DJ and Carrasco M (2014). “Attention enhances contrast appearance via increased input baseline of neural responses.” Journal of Vision 14(14): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois RL and De Valois KK (1988). Spatial vision. New York, NY, Oxford University Press; (1988) viii, 381 pp. [Google Scholar]
- Deubel H (2008). “The time course of presaccadic attention shifts.” Psychological Research 72: 630–640. [DOI] [PubMed] [Google Scholar]
- Deubel H and Schneider WX (1996). “Saccade target selection and object recognition: Evidence for a common attentional mechanism.” Vision Research 36(12): 1827–1837. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Spinelli D and Morrone MC (2001). “Automatic gain control contrast mechanisms are modulated by attention in humans: evidence from visual evoked potentials.” Vision Research 41(19): 2435–2447. [DOI] [PubMed] [Google Scholar]
- Fuller S, Park Y and Carrasco M (2009). “Cue contrast modulates the effects of exogenous attention on appearance.” Vision Research 49(14): 1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S, Rodriguez RZ and Carrasco M (2008). “Apparent contrast differs across the vertical meridian: visual and attentional factors.” Journal of Vision 8(1): 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano AM, McElree B and Carrasco M (2009). “On the automaticity and flexibility of covert attention: a speed-accuracy trade-off analysis.” Journal of Vision 9(3): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham N (1989). Visual Pattern Analyzers. New York, Oxford University Press. [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Carrasco M and Heeger DJ (2013). “Endogenous spatial attention: evidence for intact functioning in adults with autism.” Autism Research 6(2): 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Heeger DJ and Carrasco M (2013). “Exogenous spatial attention: evidence for intact functioning in adults with autism spectrum disorder.” Journal of Vision 13(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E, Rolke B and Ulrich R (2006). “Visual attention and temporal discrimination: Differential effects of automatic and voluntary cueing.” Visual Cognition 13(1): 29–50. [Google Scholar]
- Herrmann K, Montaser-Kouhsari L, Carrasco M and Heeger DJ (2010). “When size matters: attention affects performance by contrast or response gain.” Nature Neuroscience 13(12): 1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L and Dobkins KR (2005). “Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain.” Vision Research 45(9): 1201–1212. [DOI] [PubMed] [Google Scholar]
- Kinchla (1980). The Measurement of Attention Attention and Performance VIII. Nickerson R. Princeton, NJ, Psychology Press: 213–238. [Google Scholar]
- Kowler E, Anderson E, Dosher B and Blaser E (1995). “The role of attention in the programming of saccades.” Vision Research 35(13): 1897–1916. [DOI] [PubMed] [Google Scholar]
- Lennie P (2003). “The cost of cortical computation.” Current Biology 13(6): 493–497. [DOI] [PubMed] [Google Scholar]
- Li H-H, Barbot A and Carrasco M (2016). “Saccade Preparation Reshapes Sensory Tuning.” Current Biology 26(12): 1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S and Carrasco M (2006). “Sustained and transient covert attention enhance the signal via different contrast response functions.” Vision Research 46(8–9): 1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S and Carrasco M (2006). “When sustained attention impairs perception.” Nature Neuroscience 9(10): 1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S and Carrasco M (2007). “Transient covert attention does alter appearance: a reply to Schneider (2006).” Perception & Psychophysics 69(6): 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Abrams J and Carrasco M (2009). “Voluntary attention enhances contrast appearance.” Psychological Science 20(3): 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Stevens ST and Carrasco M (2007). “Comparing the time course and efficacy of spatial and feature-based attention.” Vision Research 47(1): 108–113. [DOI] [PubMed] [Google Scholar]
- Lu ZL and Dosher BA (1998). “External noise distinguishes attention mechanisms.” Vision Research 38(9): 1183–1198. [DOI] [PubMed] [Google Scholar]
- Luck SJ (2004). “Understanding awareness: one step closer.” Nature Neuroscience 7(3): 208–209. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP and Hawkins HL (1994). “Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection.” Journal of Experimental Psychology Human Perception & Performance 20(4): 887–904. [DOI] [PubMed] [Google Scholar]
- Mangun GR and Hillyard SA (1990). “Allocation of visual attention to spatial locations: tradeoff functions for event-related brain potentials and detection performance.” Perception & Psychophysics 47(6): 532–550. [DOI] [PubMed] [Google Scholar]
- Montagna B, Pestilli F and Carrasco M (2009). “Attention trades off spatial acuity.” Vision Research 49(7): 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnini A and Castet E (2007). “Spatiotemporal dynamics of visual attention during saccade preparation:independence and coupling between attention and movement planning.” Journal of Vision 7(8): 1–16. [DOI] [PubMed] [Google Scholar]
- Moore T and Zirnsak M (2017). “Neural Mechanisms of Selective Visual Attention.” Annual Review of Psychology 68: 47–72. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Denti V and Spinelli D (2002). “Color and luminance contrasts attract independent attention.” Current Biology 12(13): 1134–1137. [DOI] [PubMed] [Google Scholar]
- Morrone MC, Denti V and Spinelli D (2004). “Different attentional resources modulate the gain mechanisms for color and luminance contrast.” Vision Research 44(12): 1389–1401. [DOI] [PubMed] [Google Scholar]
- Muller HJ and Rabbitt PM (1989). “Spatial cueing and the relation between the accuracy of “where” and “what” decisions in visual search.” The Quarterly Journal of Experimental Psychology 41(4): 747–773. [DOI] [PubMed] [Google Scholar]
- Nachmias J (1967). “Effect of exposure duration on visual contrast sensitivity with square-wave gratings.” Journal of the Optical Society of America 57(3): 421–427. [Google Scholar]
- Nakayama K and Mackeben M (1989). “Sustained and transient components of focal visual attention.” Vision Research 29(11): 1631–1647. [DOI] [PubMed] [Google Scholar]
- Pestilli F and Carrasco M (2005). “Attention enhances contrast sensitivity at cued and impairs it at uncued locations.” Vision Research 45(14): 1867–1875. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Ling S and Carrasco M (2009). “A population-coding model of attention’s influence on contrast response: Estimating neural effects from psychophysical data.” Vision Research 49(10): 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestilli F, Viera G and Carrasco M (2007). “How do attention and adaptation affect contrast sensitivity?” Journal of Vision 7(7): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Listorti C and Rucci M (2013). “Microscopic Eye Movements Compensate for Nonhomogeneous Vision within the Fovea.” Current Biology 23(17): 1691–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti M, Rucci M and Carrasco C (2017). “Selective attention within the foveola.” Nature Neuroscience 20(10): 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington RW, Johnston JC and Yantis S (1992). “Involuntary attentional capture by abrupt onsets.” Perception & Psychophysics 51(3): 279–290. [DOI] [PubMed] [Google Scholar]
- Reynolds JH and Heeger DJ (2009). “The normalization model of attention.” Neuron 61(2): 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T and Desimone R (2000). “Attention increases sensitivity of V4 neurons.” Neuron 26(3): 703–714. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Hawken MJ and Shapley R (1997). “Dynamics of orientation tuning in macaque primary visual cortex.” Nature 387(6630): 281–284. [DOI] [PubMed] [Google Scholar]
- Roberts M, Ashinoff BK, Castellanos FX and Carrasco M (2017). “When attention is intact in adults with ADHD.” Psychonomic Bulletin & Review 24(6): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Cymerman R, Smith RT, Kiorpes L and Carrasco M (2016). “Covert spatial attention is functionally intact in amblyopic human adults.” Journal of Vision 16(15): 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M and Carrasco M (2012). “Rapid simultaneous enhancement of visual sensitivity and perceived contrast during saccade preparation.” Journal of Neuroscience 32(40): 13744–13752a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfs M, Jonikaitis D, Deubel H and Cavanagh P (2011;). “Predictive remapping of attention across eye movements.” Nature Neuroscience 14: 252–256. [DOI] [PubMed] [Google Scholar]
- Skottun BC, Bradley A, Sclar G, Ohzawa I and Freeman RD (1987). “The effects of contrast on visual orientation and spatial frequency discrimination: a comparison of single cells and behavior.” Journal of Neurophysiology 57(3): 773–786. [DOI] [PubMed] [Google Scholar]
- Sperling G and Melchner MJ (1978). “The attention operating characteristic: examples from visual search.” Science 202(4365): 315–318. [DOI] [PubMed] [Google Scholar]
- Talgar CP and Carrasco M (2002). “Vertical meridian asymmetry in spatial resolution: visual and attentional factors.” Psychon Bull Rev 9(4): 714–722. [DOI] [PubMed] [Google Scholar]
- Treue S (2004). “Perceptual enhancement of contrast by attention.” Trends Cogn Sci 8(10): 435–437. [DOI] [PubMed] [Google Scholar]
- Treue S and Maunsell JH (1999). “Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas.” Journal of Neuroscience 19(17): 7591–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford T and Maunsell JH (2006). “Effects of spatial attention on contrast response functions in macaque area V4.” Journal of Neurophysiology 96(1): 40–54. [DOI] [PubMed] [Google Scholar]
- Wyart V, Nobre AC and Summerfield C (2012). “Dissociable prior influences of signal probability and relevance on visual contrast sensitivity.” Proceedings of the National Academy of Sciences of the United States of America 109(9): 3593–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S and Jonides J (1996). “Attentional capture by abrupt onsets: new perceptual objects or visual masking?” Journal of Experimental Psychology Human Perception & Performance 22(6): 1505–1513. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y and Carrasco M (1998). “Attention improves or impairs visual performance by enhancing spatial resolution.” Nature 396(6706): 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y and Carrasco M (2000). “The locus of attentional effects in texture segmentation.” Nature Neuroscience 3(6): 622–627. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y and Carrasco M (2008). “The effects of transient attention on spatial resolution and the size of the attentional cue.” Perception & Psychophysics 70(1): 104–113. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y and Levy L (2003). “Transient spatial attention degrades temporal resolution.” Psychological Science 14(3): 225–231. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y and Rashal E (2010). “Precueing attention to the target location diminishes crowding and reduces the critical distance.” Journal of Vision 10(10): 16. [DOI] [PubMed] [Google Scholar]