INTRODUCTION
Perinatal asphyxia remains a frequent cause of cerebral palsy, mental retardation, learning disability, epilepsy, and death.1 The worldwide burden is 4 million newborns every year, of which one million die and an additional million have significant disabilities.2,3 Neonatal brain injury is recognized by a distinctive clinical encephalopathy that evolves from hyperexcitability to lethargy and stupor during the first 3 days of life.4,5 Large trials have shown that hypothermia therapy results in a significant reduction in death or disability with a relative risk of 0.76 (confidence interval [CI] 0.65–0.89).6–12 Hypothermia is the current standard of care for hypoxic neonatal encephalopathy (NE). However, 45% of cooled newborns have significant disabilities at 12 to 18 months of age,13 when tested by the Bayley Scales of Infant and Toddler Development gold standard neurodevelopmental tools.
Because hypothermia does not protect all affected neonates from neurocognitive delay and mental retardation, adjuvant therapies are being sought; but the real challenge is to define which neonates will need these therapies shortly after birth. Despite the complex pathophysiology related to the uncertain timing, severity, and patterns of the fetal insult,14 neonates with NE are currently viewed dichotomously15: those who do, or do not, qualify for cooling. Hypothermia is offered uniformly in a one-size-fits-all strategy based on the early neurologic examination, which has a limited predictive value, and the striking heterogeneity between the moderate and severe groups.6–12 Infants with mild NE are not currently cooled, yet 20% to 30% may have abnormal outcomes.16 Amplitude electroencephalogram17 is a good physiologic marker of neuronal integrity, but its predictive values are attenuated during hypothermia.18,19 Although brain MRI is considered the best short-term marker of early childhood outcome,20–23 the sensitivity improves beyond the first week of life. An ideal biomarker would be measured in real time and directly reflect the neurovascular unit function23 linking it to outcomes.24–26 Such a biomarker would enhance the ability to stratify the insult severity by identifying neonates with mild NE who might benefit from hypothermia and those with moderate-severe NE who need added interventions to improve outcomes. The quest for such biomarkers is still mostly research based. This review covers promising biomarkers of injury to the neurovascular unit during hypothermia therapy including (1) biomarkers of placental inflammation, (2) neuronal biomarkers, as well as (3) general inflammatory cytokines in the serum.
PLACENTAL INFLAMMATORY BIOMARKERS
The placenta mediates interactions between mother and fetus throughout gestation and provides a historical record of maternal and fetal interactions. Its fetal composition makes it an ideal storyteller of perinatal distress. In 1955, Eastman and De Leon first described the association of intrapartum infection and cerebral palsy (CP). Over the years, there has been growing evidence to support an association between placental inflammation, elevated cytokines and CP.27–29 A multitude of elegant animal studies have shown a variable response of the developing brain to inflammation, depending on the critical timing of exposure.30,31 When administered either 72 hours before or 6 hours after the insult, lipopolysaccharide (LPS) resulted in increased hypoxic-ischemic (HI) injury, in contrast to a reduced injury when administered 24 hours before the insult.30 Therapeutic hypothermia was not neuroprotective in another LPS-sensitized unilateral strokelike HI brain injury model in newborn rats.31 In a recent clinical study, postnatal sepsis was associated with increased watershed evidence of brain injury, whereas reports of isolated prenatal maternal chorioamnionitis were associated with lower incidence of MRI abnormalities in newborns with encephalopathy.32
The placental milieu can have various influences on outcomes, as well as the extent of responses to therapies, depending on the severity and timing of the inflammatory sensitization. The author and colleagues recently reported findings of a large cohort that included all 120 neonates born with a gestational age of 36 weeks or greater and a birth weight greater than 1800 g who were admitted to the neonatal intensive care unit at Parkland Hospital from January 2006 through November 2011 with evidence of perinatal acidosis and NE.33 As it was routine practice for all placentas associated with specific maternal-fetal complications of pregnancy and need for presence of the neonatal resuscitation team to be sent to pathology, placentas from all the infants during the study time period were examined. Gross and histologic examinations were reported according to the 2005 Redline classification.34 Major pathology was identified to include (1) intervillous fibrin deposition/retroplacental hemorrhage/infarction involving 20% of placental volume; (2) fetal vascular thrombo-occlusive disease with greater than 1 focus of avascular villi/thrombotic vasculopathy; (3) chorioamnionitis including a fetal response; and (4) patchy/diffuse chronic villitis. Chronic villitis was divided into low-grade (focal/multifocal villitis) and high-grade villitis (patchy/diffuse villitis). Low-grade lesions included focal villitis, a small cluster of 10 or less affected villi and multifocal villitis, and 2 or more small clusters of 10 or less affected villi. High-grade lesions included patchy villitis defined as a large cluster of more than 10 affected villi but less than 25% of the terminal villi plus diffuse villitis and the presence of extensive inflammation with multiple large clusters detected on all slides. In this high-risk inborn patient population with metabolic acidosis and encephalopathy, most (95%) had placental abnormalities and 65% met criteria for a diagnosis of a major placental pathology. There was a high incidence of inflammatory pathology observed with chorioamnionitis in 61 of 114 (54%) and chronic patchy/diffuse villitis in 13 of 114 (11%).
The frequency of occurrence increased with severity of NE. Following univariate regression analysis, which included clinical and placental variables, chronic active/patchy villitis was the only predictive variable that was significantly associated with abnormal 2-year neurodevelopmental outcomes after hypothermia (odds ratio 9.29; 95% CI 1.11–77.73). These findings suggest an important contribution by placental inflammatory mechanisms to the severity of the asphyxia and therapeutic responses following hypothermia.
SERUM BIOMARKERS OF THE NEUROVASCULAR UNIT
The neurovascular unit includes the blood-brain barrier (BBB), a key interface of molecular and cellular exchange between blood and neural tissues.19 The BBB is dynamic and can be modified by circulating factors secreted by microglia or transferred from distant organs.35 Microglia are activated in the setting of HI encephalopathy (HIE) and develop macrophagelike capabilities, including phagocytosis, cytokine and chemokine production, antigen presentation, and the release of matrix metalloproteinases that weaken the BBB. As a result, peripheral leukocytes infiltrate into the brain, leading to increased inflammation and neuronal injury. Commonly known factors upregulated after hypoxia can impair BBB permeability, such as interleukins (IL-1α, IL-1β, IL-6), tumor necrosis factor α (TNFα), free radicals, and nitric oxide, to list a few.19 Multiple organ dysfunction is an integral part of HIE; therefore, multiple organs, in addition to the leaky BBB, can contribute to the inflammatory response.36
Brain-Specific Biomarkers
The microglia and astrocytes from the neurovascular unit can further produce glial fibrillary acidic protein (GFAP), ubiquitin carboxyl-terminal hydrolase L1 (UCHL-1), S100B, neuron-specific enolase (NSE), as well as cytokines, chemokines, and other factors.19,37 Table 1 summarizes reports of neuronal biomarkers detected in the serum of neonates with HIE. All of the studies,38–45 although novel, represent small pilot studies and are not powered to detect long-term outcome predictions. GFAP is an intermediate filament protein released from astrocytes into the blood on astrocyte death and has been correlated with poor outcomes in adult patients after stroke, cardiac arrest, extracorporeal membrane oxygenation, or traumatic brain injury.43 Serum GFAP has been recently reported by Ennen and colleagues45 to be significantly elevated in newborns with NE undergoing hypothermia therapy when compared with controls. A GFAP threshold level greater than 0.15 ng/mL following hypothermia therapy was associated with an abnormal brain MRI. UCH-L1 is a neuron-specific cytoplasmic enzyme and marker of neuronal apoptosis that is concentrated in dendrites. Serum UCH-L1 reflects the extent of neuronal injury because it is expressed in neurons and then released into the circulation after breakdown of the BBB and is easily measured.44 In another study, UCH-L1 serum values were greater than 100 ng/mL in neonates with NE who subsequently died.39 Additionally, serum NSE cutoff point concentrations of 45.4 mcg/L have also been associated with severity of NE and could help distinguish infants with poor outcomes.46
Table 1.
Biomarkers of the neurovascular unit: sources, functions, and conditions detected focusing on hypoxic-ischemic encephalopathy
| Neurovascular Unit Biomarkers | Sources and Functions | Conditions Where Detected | Neonatal HIE Studies |
|---|---|---|---|
| GFAP | |||
| Ennen et al,45 2011 Massaro et al,41 2013 Chalak et al,13,38 2014 |
Intermediate filament protein from astrocyte
|
In stroke, cardiac arrest, ECMO, TBI, HIE |
|
| UCH-L1 | |||
| Douglas-escobar et al,39 2010 Massaro et al,41 2013 Chalak et al,13,38 2014 |
Neuronal protein
|
In subarachnoid hemorrhage, TBI, HIE |
|
| SB100 | |||
| Gazzalo 2004 and 2009; Risso 2011 Massaro et al,40,42 2012, 2014 |
Protein binding calcium
|
In newborns higher, decrease with age, increased with HIE |
|
| NSE | |||
| Celtik et al,46 2004 Massaro et al,40,42 2012, 2014 |
Glycolytic enzyme in neurons
|
Cardiac arrest, TBI, stroke, cardiac surgery, HIE |
|
Abbreviations: CSF, cerebrospinal fluid; ECMO, extracorporeal membrane oxygenation; TBI, traumatic brain injury
Brain-specific biomarkers in the serum and neurodevelopmental outcomes after hypothermia
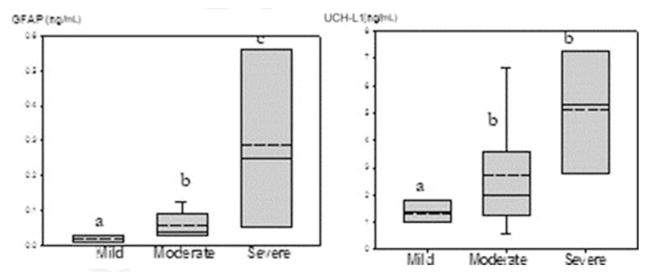
Particular emphasis is placed on the review of relatively more brain-specific GFAP and UCHL-1, whereby limited long-term outcomes were initially reported beyond death and/or MRI abnormalities. The author and colleagues recently conducted a prospective pilot cohort study to assess GFAP, along with UCH-L1, and cytokines in the serum from the cord arterial blood as well as an indwelling umbilical artery at 6 to 24, 48, 72, and 78 hours of age. Neurodevelopmental outcomes (Bayley Scales of Infant and Toddler Development III) were performed at 18 to 24 months. The umbilical arterial cord serum GFAP and ubiquitin levels correlated with severity of NE, with higher levels in moderate to severe NE, as compared with those not cooled with mild NE.38 At birth, serum GFAP and UCH-L1 were increased with the severity of NE as seen in Fig. 1 (P<.001). UCHL-1 decreased after the first 6 to 24 hours of hypothermia compared with the cord blood values at birth, possibly reflecting the effect of hypothermia on this specific neuronal marker of apoptosis. In contrast, serial GFAP values remained elevated in neonates with moderate to severe NE who were undergoing hypothermia. In addition, GFAP levels of greater than 0.05 ng/mL beyond 72 hours of age were associated with abnormal Bayley III neurodevelopmental outcomes at 24 months of age.38 Another recent study reported UCH-L1 to also be elevated in the umbilical arterial cord plasma, whereas GFAP was significantly higher at 72 hours in cooled infants who developed adverse outcomes.41 The sustained increase in GFAP over the duration of hypothermia could result from its multiple functions, which also involve repair and growth beyond the acute injury phase.
Fig. 1.
Umbilical cord plasma GFAP and UCH-L1 and severity of encephalopathy at birth. A box and whisker plot gives median as solid line, mean as dotted line, and 25th and 75th quartiles as lower and upper borders. Analyses include neonates with mild, moderate, and severe encephalopathy. P = .001 (GFAP) and P = .03 (UCH-L1) by Jonckheere-Terpstra test. a, b, and c denote significant differences. (From Chalak LF, Sanchez PJ, Adams-Huet B, et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatrics 2014;164 (3):468; with permission.)
GENERAL INFLAMMATORY BIOMARKERS
The term cytokine encompasses a variety of proteins including interleukins, colony-stimulating factors, interferons (IFNs), TNF, and chemokines47 which link the neural, endocrine, and immune systems.48 Cytokines are mediators in the common pathways associated with perinatal brain injury induced by a variety of insults.49–53 Cytokines are chemotactic for blood-borne neutrophils and monocytes/macrophages, potentially increasing leukocyte recruitment as well as directly amplifying inflammatory cascades in the injured central nervous system.27 Hypoxia-asphyxia and infection seem to potentiate the systemic inflammatory response associated with elevated cytokines.28,54,55 Serum cytokines from the neonatal screening were reported to be elevated in infants who later developed CP.27 Elevated concentrations of IL-6 and IL-8 have been demonstrated in the CSF and serum of asphyxiated full-term infants.56 Higher concentrations of IL-1β, IL-6, TNF-α, and IL-8 in the blood of neonates with HIE have also been associated with abnormal neurodevelopmental outcomes.57,58 Before the hypothermia era, meta-analysis highlighted both serum IL-1β and serum IL-6, when measured before 96 hours in infants with encephalopathy,59 as biomarkers predictive of abnormal outcomes.
Inflammatory Cytokines and Neurodevelopmental Outcomes After Hypothermia Therapy
The focus of this current report is to review inflammatory biomarkers following implementation of hypothermia therapy for newborns of HIE. The author and colleagues recently reported in a cohort of 30 newborns with encephalopathy that IL-1, IL-6, IL-8, TNF, and INF were elevated at 6 to 24 hours after birth in the infants with later abnormal neurologic outcomes at 18 to 24 months.38 The author and colleagues and others60 have further investigated the temporal relationships of biomarkers in injury and recovery and the variability of those phases during hypothermia. Some biomarkers, such as IL-1, IL-6, IL-8, and MCP1, were found to have early peaks within the first 24 to 48 hours of life, as compared with others, such as vascular endothelial growth factor (VEGF) and MIP1α, which peaked later, around 72 hours. The recent study by Celtik and colleagues46 found no differences between the effect of total and selective head cooling on inflammatory biomarkers after HIE.
SUMMARY
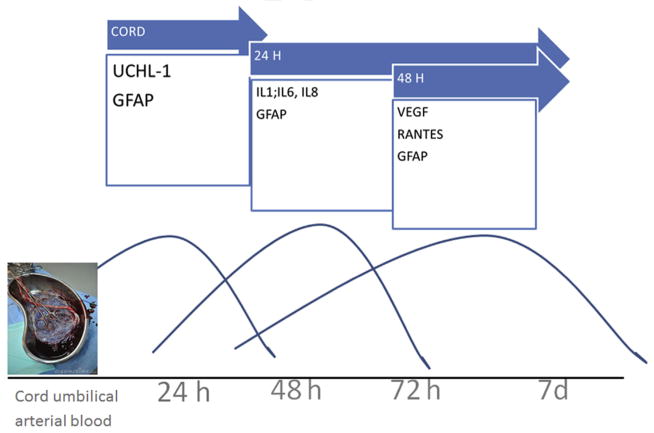
Understanding of the modulation of serum cytokines and neuronal and placental biomarkers is needed to better delineate the injury-repair pathways and responses to neuroprotective therapies. Given the complexity of the presentation and spectrum of NE/HIE, it is unlikely that a single biomarker at birth will be able to predict clinical outcomes following therapy (Fig. 2). Future neuroprotective strategies targeting inflammation will likely benefit from development of such biomarkers, as proxy to long-term outcomes, to help evaluate therapeutic efficacy and responses in real time.
Fig. 2.
Biomarkers temporal profile changes during hypothermia therapy in the first 72 hours of life.
KEY POINTS.
Hypothermia is a recognized standard-of-care neuroprotective therapy for newborns with hypoxic-ischemic encephalopathy, but about 45% of infants have abnormal outcomes despite treatment.
Biomarkers could help the bedside clinician identify responders and nonresponders to hypothermia who could benefit from added neuroprotective strategies.
The author’s observations suggest an important role of the placenta through inflammatory mechanisms to the severity of the hypoxic-asphyxial insult and therapeutic responses following hypothermia therapy.
Using a serum panel of inflammatory and neuronal biomarkers, rather than a single biomarker, seems the most promising once validated in large cohorts.
Best practices.
What is the current practice?
Hypothermia is the standard of care for neuroprotective therapy for newborns with HIE, but about 45% of infants have abnormal outcomes despite treatment.
Biomarkers are being evaluated in research studies to help the bedside clinician identify responders and nonresponders to hypothermia who could benefit from added neuroprotective strategies.
What changes in current practice are likely to improve outcomes?
Placental examination for neonates with NE, if results were available in a timely manner, could identify a subgroup of patients who might benefit from strategies targeting antiinflammatory therapies in addition to hypothermia.
Although GFAP and UCHL1 seem to be promising as brain-specific biomarkers, there is no currently available biomarker to be recommended for clinical use.
Future serum biomarker studies will need to be conducted in multiple centers to have sufficient patient numbers for large dataset validation for clinical practice.
Footnotes
Disclosure Statement: The author has nothing to disclose.
References
- 1.Levene ML, Kornberg J, Williams TH. The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev. 1985;11(1):21–6. doi: 10.1016/0378-3782(85)90115-x. [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 3.Pryds O, Greisen G, Lou H, et al. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990;117(1 Pt 1):119–25. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 4.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 5.Miller SP, Latal B, Clark H, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190(1):93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 7.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157(3):367–72. 372.e1–3. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Simbruner G, Mittal RA, Rohlmann F, et al. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO network RCT. Pediatrics. 2010;126(4):e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 11.Spitzmiller RE, Phillips T, Meinzen-Derr J, et al. Amplitude-integrated EEG is useful in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischemic encephalopathy: a meta-analysis. J Child Neurol. 2007;22(9):1069–78. doi: 10.1177/0883073807306258. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 13.Chalak LF, DuPont TL, Sanchez PJ, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol. 2014;34(8):629–33. doi: 10.1038/jp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351(19):1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 15.Ferriero DM, Bonifacio SL. The search continues for the elusive biomarkers of neonatal brain injury. J Pediatr. 2014;164(3):438–40. doi: 10.1016/j.jpeds.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Dupont TL, Chalak LF, Morriss MC, et al. Short-term outcomes of newborns with perinatal acidemia who are not eligible for systemic hypothermia therapy. J Pediatr. 2012;162(1):35–41. doi: 10.1016/j.jpeds.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shalak LF, Laptook AR, Velaphi SC, et al. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111(2):351–7. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- 18.Thoresen M, Hellstrom-Westas L, Liu X, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126(1):e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 19.McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int. 2012;2012:561494. doi: 10.1155/2012/561494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19(1):143–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics. 2010;125(2):e382–95. doi: 10.1542/peds.2009-1046. [DOI] [PubMed] [Google Scholar]
- 22.Rutherford M, Srinivasan L, Dyet L, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36(7):582–92. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159(5):851–8. e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratzer I, Chip S, Vexler ZS. Barrier mechanisms in neonatal stroke. Front Neurosci. 2014;8:359. doi: 10.3389/fnins.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juul SE, Ferriero DM. Pharmacologic neuroprotective strategies in neonatal brain injury. Clin Perinatol. 2014;41(1):119–31. doi: 10.1016/j.clp.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson KB, Dambrosia JM, Grether JK, et al. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44(4):665–75. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 28.Shalak LF, Laptook AR, Jafri HS, et al. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics. 2002;110(4):673–80. doi: 10.1542/peds.110.4.673. [DOI] [PubMed] [Google Scholar]
- 29.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 30.Eklind S, Mallard C, Arvidsson P, et al. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58(1):112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 31.Osredkar D, Thoresen M, Maes E, et al. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation. 2014;85(4):567–72. doi: 10.1016/j.resuscitation.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Jenster M, Bonifacio SL, Ruel T, et al. Maternal or neonatal infection: association with neonatal encephalopathy outcomes. Pediatr Res. 2014;76(1):93–9. doi: 10.1038/pr.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mir IN, Johnson-Welch SF, Nelson DB, et al. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849, e1–8. doi: 10.1016/j.ajog.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Redline RW, Heller D, Keating S, et al. Placental diagnostic criteria and clinical correlation - a workshop report. Placenta. 2005;26:S114–7. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 36.Vela JM, Molina-Holgado E, Arevalo-Martin A, et al. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20(3):489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- 37.Mir IN, Chalak LF. Serum biomarkers to evaluate the integrity of the neurovascular unit. Early Hum Dev. 2014;90(10):707–11. doi: 10.1016/j.earlhumdev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Chalak LF, Sanchez PJ, Adams-Huet B, et al. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164(3):468–74. e1. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas-Escobar M, Yang C, Bennett J, et al. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr Res. 2010;68(6):531–6. doi: 10.1203/PDR.0b013e3181f85a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massaro AN, Chang T, Kadom N, et al. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J Pediatr. 2012;161(3):434–40. doi: 10.1016/j.jpeds.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massaro AN, Jeromin A, Kadom N, et al. Serum biomarkers of MRI brain injury in neonatal hypoxic ischemic encephalopathy treated with whole-body hypothermia: a pilot study. Pediatr Crit Care Med. 2013;14(3):310–7. doi: 10.1097/PCC.0b013e3182720642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massaro AN, Chang T, Baumgart S, et al. Biomarkers S100B and neuron-specific enolase predict outcome in hypothermia-treated encephalopathic newborns*. Pediatr Crit Care Med. 2014;15(7):615–22. doi: 10.1097/PCC.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelinka LE, Kroepfl A, Leixnering M, et al. GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J Neurotrauma. 2004;21(11):1553–61. doi: 10.1089/neu.2004.21.1553. [DOI] [PubMed] [Google Scholar]
- 44.Papa L, Akinyi L, Liu MC, et al. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med. 2010;38(1):138–44. doi: 10.1097/CCM.0b013e3181b788ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ennen CS, Huisman TA, Savage WJ, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205(3):251, e1–7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Celtik C, Acunas B, Oner N, et al. Neuron-specific enolase as a marker of the severity and outcome of hypoxic ischemic encephalopathy. Brain Dev. 2004;26(6):398–402. doi: 10.1016/j.braindev.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics–an update. Eur J Pharmacol. 2008;579(1–3):1–12. doi: 10.1016/j.ejphar.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 48.Rostene W, Dansereau MA, Godefroy D, et al. Neurochemokines: a menage a trois providing new insights on the functions of chemokines in the central nervous system. J Neurochem. 2011;118(5):680–94. doi: 10.1111/j.1471-4159.2011.07371.x. [DOI] [PubMed] [Google Scholar]
- 49.Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35(4):643–63. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster-Barber A, Dickens B, Ferriero DM. Human perinatal asphyxia: correlation of neonatal cytokines with MRI and outcome. Dev Neurosci. 2001;23(3):213–8. doi: 10.1159/000046146. [DOI] [PubMed] [Google Scholar]
- 51.Elovitz MA, Brown AG, Breen K, et al. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011;29(6):663–71. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the pre-term newborn. J Child Neurol. 2009;24(9):1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shalak LF, Perlman JM. Infection markers and early signs of neonatal encephalopathy in the term infant. Ment Retard Dev Disabil Res Rev. 2002;8(1):14–9. doi: 10.1002/mrdd.10006. [DOI] [PubMed] [Google Scholar]
- 54.Ellison VJ, Mocatta TJ, Winterbourn CC, et al. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57(2):282–6. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- 55.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112(1 Pt 1):176–80. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 56.Savman K, Blennow M, Gustafson K, et al. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43(6):746–51. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 57.Chiesa C, Pellegrini G, Panero A, et al. Umbilical cord interleukin-6 levels are elevated in term neonates with perinatal asphyxia. Eur J Clin Invest. 2003;33(4):352–8. doi: 10.1046/j.1365-2362.2003.01136.x. [DOI] [PubMed] [Google Scholar]
- 58.Bartha AI, Foster-Barber A, Miller SP, et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56(6):960–6. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 59.Ramaswamy V, Horton J, Vandermeer B, et al. Systematic review of biomarkers of brain injury in term neonatal encephalopathy. Pediatr Neurol. 2009;40(3):215–26. doi: 10.1016/j.pediatrneurol.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins DD, Rollins LG, Perkel JK, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2012;32(10):1888–96. doi: 10.1038/jcbfm.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]