Abstract
Hesperetin, a naturally occurring citrus flavanone of the bioactive substance, possesses different pharmacological and biochemical activities including anti-cancer and anti-oxidants effect. The aim of the study to investigate that hesperetin on abnormalities of glycoconjugates (protein bound hexose, hexosamine, total sialic acid and fucose), histology (PAS staining) and immunoexpression of cytokeratin during 7,12-dimethylbenz(a)anthracene (DMBA) induced hamster buccal pouch (HBP) carcinogenesis. Oral tumors were developed in the buccal pouches of male golden Syrian hamsters by topical application of 0.5% DMBA thrice a week for 10 weeks and developed morphological alterations depicted as hyperplasia, dysplasia and well-differentiated squamous cell carcinoma formation with noticeable abnormalities of glycoconjugates and cytokeratin. The protective effect of hesperetin against DMBA was evaluated by assessing immunohistochemical expression, histological sections of buccal tissues and the levels of glycoconjugates in the buccal mucosa and plasma were analyzed. Hesperetin administrated orally at a dose of 20 mg/kg b.w. to hamsters treated with DMBA, significantly reduced the status of glycoconjugates and cytokeratin to the near normal range. Overall findings accomplished that hesperetin protects cell surface glycoconjugates abnormalities in DMBA induced HBP carcinogenesis.
Keywords: Oral cancer, DMBA, Hesperetin, Glycoconjugates, Cytokeratin
Introduction
Oral squamous cell carcinoma (OSCC), a malignant neoplasm is a part of head and neck cancer and the cancerous tissue arises from the oral cavity. It is the 5th most common cancer and major oncological problem in the world. This type of malignant neoplasm refers to the lip and floor of the mouth. Several risk factors are associated with oral cancer with high morbidity and mortality in Asian countries like India, Sri Lanka and Bangladesh etc., where it accounts for 45–50% of all types of cancer. The major etiological factors are betel quid, smoking, tobacco products and alcohol consumption [1].
7,12-dimethylbenz(a)anthracene (DMBA) is a polycyclic aromatic hydrocarbon (PAH) and an organ specific potent procarcinogen generally used as a chemical carcinogen to stimulate experimental oral carcinogenesis. DMBA undergoes activation by phase I enzymes to form diolepoxide, oxidizing both purine bases of DNA causing mutation [2]. DMBA induced HBP carcinogenesis is considered as an ideal experimental model to study the anticancer potential of natural products.
Glycoconjugates are complex molecules such as peptidoglycans, glycopeptides, glycoproteins, glycolipids and lipopolysaccharides, which are found outside of the cell membranes [3]. These cell surface molecules play a major role in tissue organization with several biological functions including cell differentiation, cell–cell communication, cell adhesion, cell proliferation and differentiation. Abnormal glycoconjugates levels were documented in several malignant cancers including oral carcinomas [4]. Elevated sialylation and fucosylation are well documented in malignant cell transformation [5]. Cytokeratin is the epithelial cell specific protein that is a sensitive marker in various pathological conditions including oral cancer and a useful tool in diagnostics oncology [6].
Researchers have focused on herbal plants has improved in recent years due to health benefits, particularly as a defense against a variety of disorders such as suppression or prevention of insidious carcinoma. Flavonoids are the group of polyphenolic compounds synthesized by plants as secondary metabolites. Hesperetin (3′,5,7-trihydroxyl-4′-methoxyl flavanone) is a natural predominant bioactive flavanone, a subclass of flavonoids, which are abundantly found in citrus fruits [7]. It exhibits antioxidant [8], antiproliferative, antilipidperoxidative [9], antihypertensive, antiatherogenic [10], antiinflammatory [11], antirheumatic [12], immunomodulatory [13] and antitumor activity [14]. The present study was undertaken to investigate the membrane stabilizing effects of hesperetin on the levels of glycoconjugates, immunohistochemistry of cytokeratin and histological changes in the buccal tissues of DMBA induced OSCC.
Materials and Methods
Chemicals
The DMBA (Purity ≥ 95%, CAS No: 57-97-6) and hesperetin (Chemical catalog: W431300-5G, CAS NO: 69097-99-0, Purity: ≥ 95%) were obtained from Sigma-Aldrich Chemical Pvt. Ltd., Bangalore, India. Primary antibody Cytokeratin with secondary antibody Goat anti-rabbit IgG-HRP was obtained from Santa Cruz Biotechnology, California, USA. All other chemicals used were of analytical grade, purchased from Hi-media Laboratories Pvt. Ltd. Mumbai, India.
Animals
Male golden Syrian hamsters (weighing 80–120 g) were acquired from the National Institute of Nutrition (NIN), Hyderabad, India. They were housed in propylene cages (47 cm × 34 cm × 20 cm) with provided standard pellet diet and water ad libitum. The hamsters were maintained under controlled environmental conditions with 12-h L/D cycles, humidity (55 ± 5%) and temperature (27 ± 2 °C).
Drug Treatment
Hesperetin powder was suspended in 0.1% carboxymethylcellulose (CMC) and each hamster received 1 mL as suspension.
Tumors Induction
Tumors were induced into HBP by topical application of 0.5% DMBA in liquid paraffin three times a week for 10 weeks.
Experimental Design
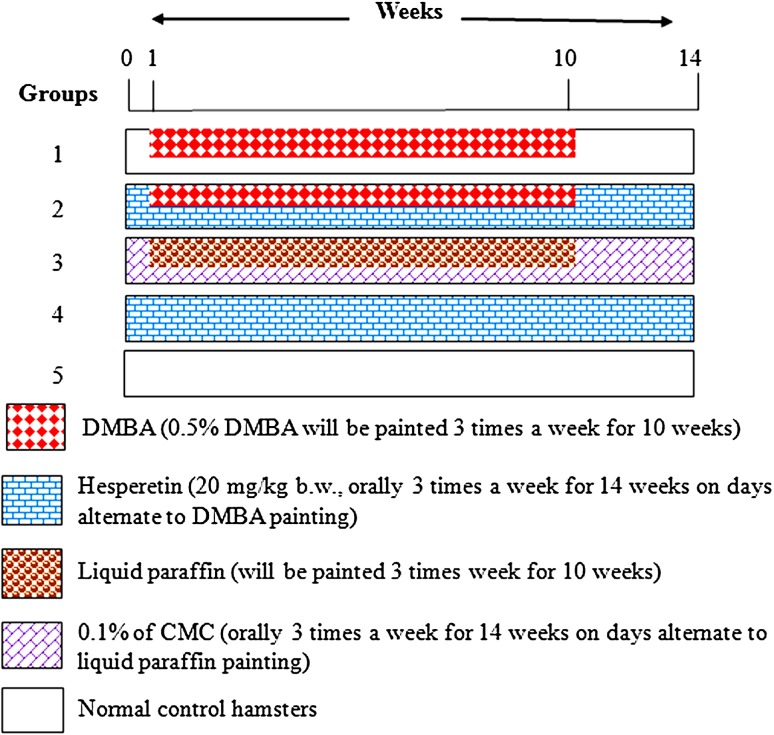
A total of 30 hamsters were randomized into five groups of six animals in each group. Figure 1 was showed the experimental design. The experiment was terminated at the end of 14th weeks and all hamsters were sacrificed by cervical dislocation.
Fig. 1.
Schematic representation of the experimental design
Samples Collection
Blood samples were collected and then centrifuged at 1000×g for 15 min for the separation of plasma. Tissue samples from the hamsters were washed with ice-cold saline and homogenized to use the suitable buffer in a homogenizer with Teflon pestle and used for biochemical estimations.
Biochemical Analysis
The precipitate obtained after treating the plasma with 95% ethanol was used for the estimation of protein bound hexose and hexosamine. The defatted buccal mucosa tissues obtained after treating the tissues with methanol and chloroform was used for the estimation of glycoproteins. The protein bound hexose in plasma and defatted buccal mucosa tissues were estimated by the method of Niebes [15]. The protein bound hexosamine in plasma was estimated by the method of Wagner [16]. The total sialic acid in plasma and defatted buccal mucosa tissues were estimated by the method of Warren [17]. Fucose in plasma and buccal mucosa tissue were estimated by the method of Dische [18]. Proteins were estimated by the method of Lowry et al. [19].
Histopathological Study with Periodic Acid-Schiff (PAS) Staining
For histopathological analysis of buccal tissues were sliced and immersed in 10% formalin solution for fixation, dry with graded ethanol solution since 50–100% and then embedded in paraffin. Sections of 3–5 μm in thickness used for histological studies, stained with 0.1% of periodic acid staining for 15 min, at 50 °C, then the slides were washed in running tap water, counterstained with hematoxylin, dehydrated with ethanol, cleared in xylene and mounted with resinous medium and observed under the microscope.
Immunohistochemical (IHC) Staining
Paraffin-embedded tissue sections were dewaxed and rehydrated through graded ethanol with distilled water. Endogenous peroxidase was blocked by incubation with 3% H2O2 in methanol for 10 min. The tissue sections were then incubated with primary antibody cytokeratin (1:50 diluted PBS) overnight at 4 °C. The bound primary antibody was detected by incubation with the secondary antibody conjugated to horseradish peroxidase (HRP) before 30 min at room temperature. To achieve color development, sections were incubated with 3,3′-diaminobenzidine (DAB) for 20 min using the labeled polymer HRP secondary detection kit and counterstained with hematoxylin following Dibutylphthalate Polystyrene Xylene (DPX) mount was performed. Controls were obtained replacing primary antibody with Phosphate buffered saline (PBS). Immunoreactivities were regarded as positive if the apparent staining were detected in the tissues when observed under high-power Microscope (Olympus Bx 40-Japan) attached to a digital camera.
Statistical Analysis
Statistical analysis was performed using SPSS 17 (SPSS, Inc., Chicago) statistical package. The data are expressed as the mean ± standard deviation (SD). One way analysis of variance (ANOVA) followed by Duncan Multiple Range Test (DMRT) comparisons a method was used to correlate difference between the variables. Data are considered statistically significant if p < 0.05.
Results
The levels of cell surface glycoconjugates (protein bound hexose, hexosamine, total sialic acid and fucose) in the plasma of normal control and experimental hamsters depicted in Table 1. The levels of glycoconjugates in the plasma were significantly increased in hamsters treated with DMBA alone (Group 1). Oral pre-administration of hesperetin (20 mg/kg b.w.) to DMBA treated hamsters (Group 2) improved the levels of glycoconjugates (protein bound hexose, hexosamine, total sialic acid and fucose) to the near normal range. No significant changes of plasma glycoconjugates were noticed in the hesperetin alone (Group 3) and CMC + Liquid paraffin (Group 4) treated hamsters as compared to normal control hamsters (Group 5).
Table 1.
Protein bound hexose, protein bound hexosamine, total sialic acid and fucose in the plasma of normal control and experimental hamsters in each group
| Groups | Treatment/parameters | Protein bound hexose (mg/dL) | Protein bound hexosamine (mg/dL) | Total sialic acid (mg/dL) | Fucose (mg/dL) |
|---|---|---|---|---|---|
| 1 | DMBA alone | 137.31 ± 10.50b | 119.34 ± 9.13b | 82.41 ± 6.30b | 18.56 ± 1.41b |
| 2 | DMBA + Hesperetin (20 mg/kg b.w.) | 102.07 ± 7.77c | 68.75 ± 5.23c | 34.99 ± 2.66c | 6.78 ± 0.51c |
| 3 | Hesperetin alone | 88.97 ± 6.77a | 82.11 ± 6.25a | 45.96 ± 3.50a | 8.61 ± 0.66a |
| 4 | CMC + Liquid paraffin | 88.52 ± 6.74a | 82.01 ± 6.24a | 45.73 ± 3.48a | 8.47 ± 0.64a |
| 5 | Normal control | 89.06 ± 6.77a | 82.87 ± 6.31a | 46.02 ± 3.50a | 8.10 ± 0.25a |
Values are expressed as mean ± SD for six animals in each group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT)
The levels of cell surface glycoconjugates (protein bound hexose, total sialic acid and fucose) in the buccal mucosa of normal control and experimental hamsters depicted in Table 2. The levels of glycoconjugates in the buccal mucosa were significantly increased in hamsters treated with DMBA alone (Group 1). Oral pre-administration of hesperetin (20 mg/kg b.w.) to DMBA treated hamsters (Group 2) altered the levels of glycoconjugates (protein bound hexose, total sialic acid and fucose) to the near normal range. No significant changes of buccal mucosa glycoconjugates were noticed in the hesperetin alone (Group 3) and CMC + Liquid paraffin (Group 4) treated hamsters as compared to normal control hamsters (Group 5).
Table 2.
Protein bound hexose, total sialic acid and fucose in the buccal mucosa of normal control and experimental hamsters in each group
| Groups | Treatment/parameters | Protein bound hexose (mg/dL) | Total sialic acid (mg/dL) | Fucose (mg/dL) |
|---|---|---|---|---|
| 1 | DMBA alone | 155.84 ± 11.92b | 32.96 ± 1.25b | 26.27 ± 2.00b |
| 2 | DMBA + hesperetin (20 mg/kg b.w.) | 98.64 ± 7.14c | 13.18 ± 1.10c | 13.02 ± 1.12c |
| 3 | Hesperetin alone | 106.06 ± 8.14a | 15.28 ± 1.16a | 14.95 ± 1.44a |
| 4 | CMC + liquid paraffin | 108.95 ± 8.15a | 15.07 ± 1.15a | 14.83 ± 1.12a |
| 5 | Normal control | 107.86 ± 8.21a | 15.55 ± 1.18a | 15.01 ± 1.05a |
Values are expressed as mean ± SD for six animals in each group. Values not sharing a common superscript differ significantly at p < 0.05 (DMRT)
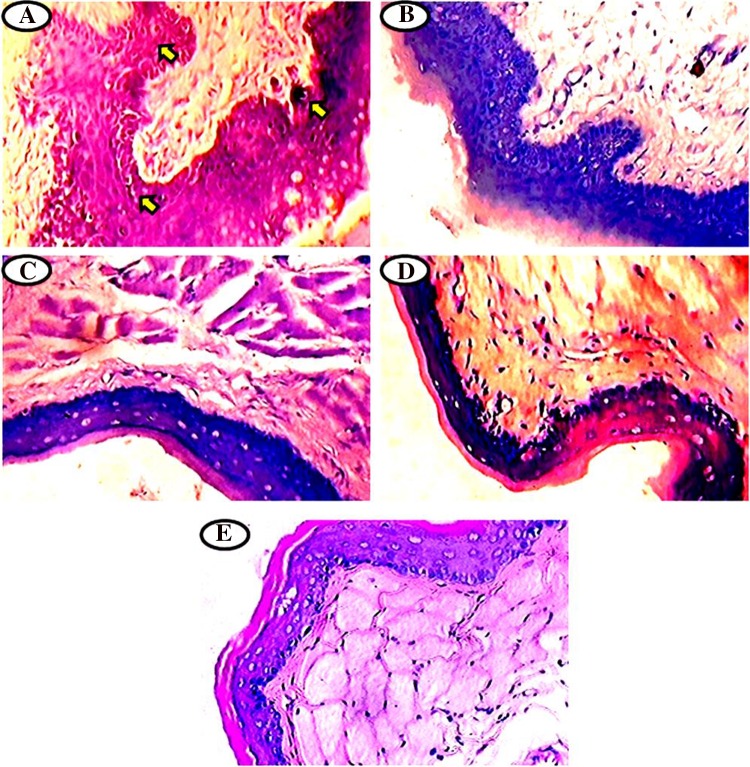
The glycoconjugates expression pattern was analyzed using PAS staining in the buccal mucosa of normal control and experimental hamsters in each group (Fig. 2a–e). The present study shows a clearly increased in the glycoconjugates content with keratosis, hyperplasia and dysplasia and well-differentiated tumor formation in the DMBA alone painted hamsters (Group 1, Fig. 2a). Oral pre-administration of hesperetin to DMBA treated hamsters (Group 2, Fig. 2b) normalized the levels of above glycoconjugates when compared to control. No significant difference was noticed in the expression of glycoconjugates in the hesperetin alone (Group 3, Fig. 2c), CMC + Liquid paraffin treated (Group 4, Fig. 2d) as compared to normal control hamsters (Group 5, Fig. 2e).
Fig. 2.
a–e Histochemical analysis of glycoconjugates by periodic acid Schiff’s staining (PAS) in the buccal mucosa of normal control and experimental hamsters. a Over expression of glycoconjugates in DMBA alone treated group 1 hamsters. b Lowered expression of glycoconjugates in DMBA + hesperetin (20 mg/kg bw) treated group 2 hamsters. c–e Glycoconjugates expression pattern was normal in the hesperetin alone group 3, CMC + liquid paraffin group 4 and normal control group 5 hamsters
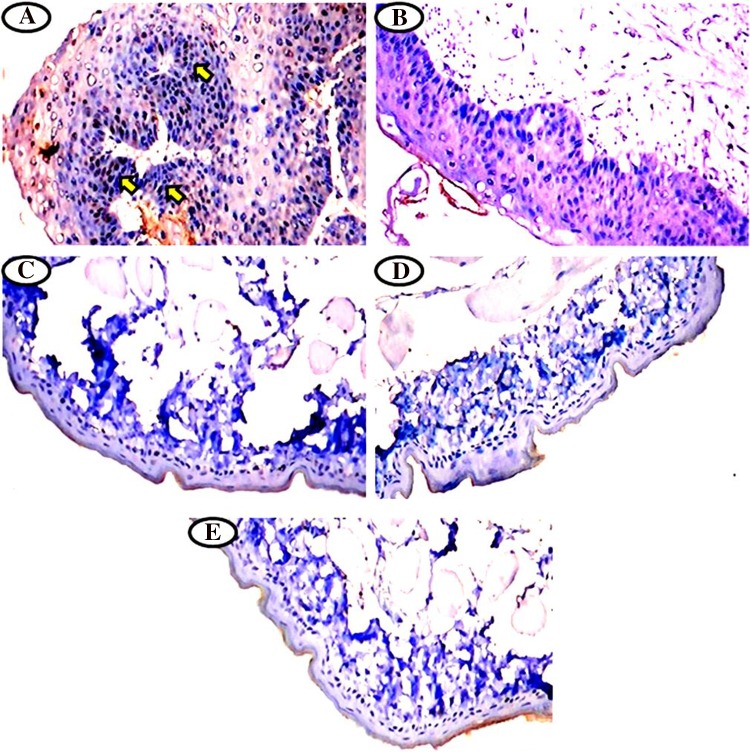
Immunohistochemical expression of cytokeratin in the buccal mucosa of normal control and experimental hamsters in each group (Fig. 3a–e). Upregulated the expression of cytokeratin in hamsters treated with DMBA alone (Group 1, Fig. 3a). Oral pre-administration of hesperetin to DMBA treated hamsters significantly down regulated the expression of cytokeratin (Group 2 Fig. 3b). Cytokeratin expression was normal in hesperetin alone (Group 3, Fig. 3c), CMC + Liquid paraffin treated (Group 4, Fig. 3d) as compared to normal control hamsters (Group 5, Fig. 3e).
Fig. 3.
a–e Immunohistochemical staining of cytokeratin expression observed in the buccal mucosa of normal control and experimental hamsters. a Higher expression of cytokeratin in DMBA alone treated group 1 hamsters. b Moderate cytokeratin expression in DMBA + hesperetin (20 mg/kg bw) treated group 2 hamsters. c–e Normal levels of cytokeratin expression in hesperetin alone group 3, CMC + liquid paraffin group 4 and normal control group 5 hamsters
Discussion
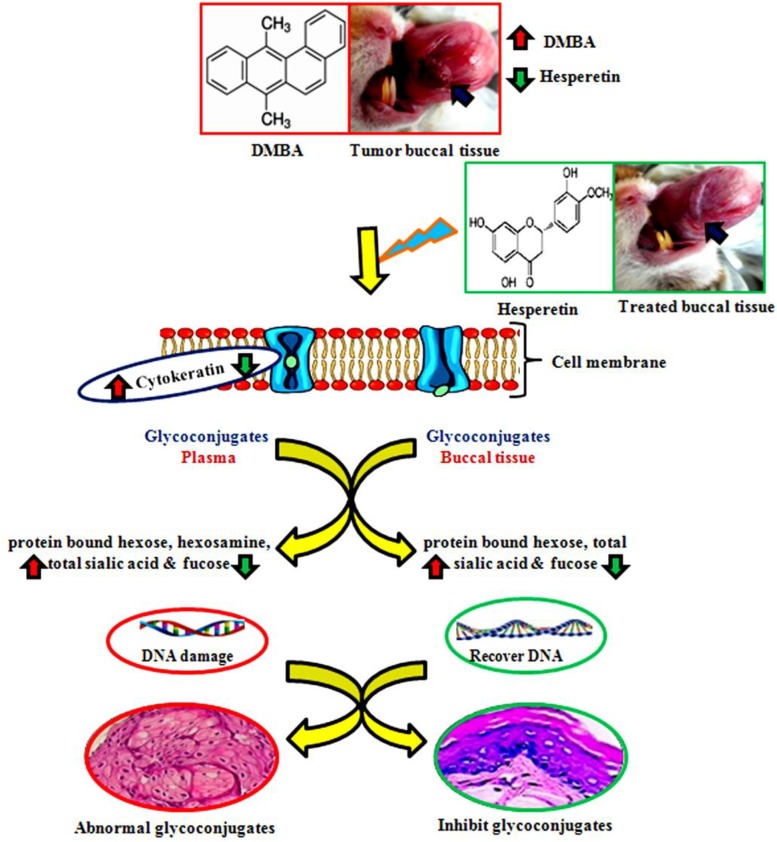
Glycoproteins are conjugated proteins that have covalently linked carbohydrate chains which contribute to the structure of extracellular matrix in animal cells. Carbohydrates are N-linked to asparagine (N-glycans) or O-linked to serine/threonine (O-glycans) of the protein molecules. Various cancer studies revealed elevated levels of glycoproteins in the neoplastic cells and altered cell surface glycoconjugates leading to uncontrolled growth and metastasis and inflammation [20]. Elevated levels of glycoprotein components could be a consequence of impaired carbohydrate metabolism in tumor tissues. Previous studies demonstrated that tissue and circulatory glycoconjugates levels are useful biomarkers of oral carcinogenesis [21]. A positive correlation between tumor tissue glycoconjugates and clinical stages of the tumor are well known in human and experimental oral carcinogenesis [22]. Toxic metabolites of DMBA (diolepoxide) are capable of binding to adenine residues of DNA forming adducts, which initially causing oxidative stress leads to chromosomal damage and finally malignancy [23]. This study was established by an in vivo model of HBP and hesperetin administrations to tumor bearing hamsters effectively modulate the glycoconjugates levels are shown in Fig. 4.
Fig. 4.
Probable mechanism essential the DMBA induced hamster buccal pouch carcinogenesis with defense mechanism through hesperetin. Red arrow indicates the abnormalities of DMBA induced oral carcinogenesis. Green arrow indicates the pharmacological effects of hesperetin
Cytokeratin is family of intermediate filament proteins that occurs most abundantly in the cytoskeleton of epithelial cells, which are responsible for cellular integrity. It is a useful marker for detection and diagnosis of various types of cancer including oral cancer. The major function of cytokeratin is to protect epithelial cells from various stresses and involved in cell signaling, apoptosis etc. In various types of cancers including cervical, laryngeal, oesophageal, lung and oral squamous cell carcinoma, the cytokeratin expression was high in cancerous tissue compared with normal counterpart and it plays a major role in the progression of tumor tissues [24]. Rajkamal et al. [25] documented that overexpression of cytokeratin in DMBA induced HBP carcinogenesis. Our results corroborate these findings. Administration of hesperetin to DMBA treated hamsters reduces the expression of cytokeratin in HBP carcinogenesis.
Protein-bound hexose contributes hydrophilic nature to the cell membrane and cationic charges to make cell membrane polarized. Altered glycosylation patterns by glycosyltransferase cause abnormal levels of glycoconjugates which indicates malignant transformation that is subsequently shed into systemic circulation. Fucose plays a significant role in many diseases including cancer and its incidence [26]. Bose et al. [27] reported tumor cell surface with increased fucose levels and decreased cell adhesion molecules. Elevated fucose covalently linked to lipids/proteins, which play a crucial role in cell surface of various cancer cells [28]. Sialic acid is a terminal sugar of the carbohydrate chains of glycoconjugates with several biological functions including adhesiveness and cellular invasiveness. An increased concentration of sialic acid on the tumor cell surface, play a crucial role in the metastasis by escaping from apoptosis, ability to increase vascular endothelial adherence and decreased the immunogenicity of tumor cells by host protection system [29]. Vinothkumar et al. [30] documented that increased protein bound hexose, hexosamine, fucose and sialic acid content probably due to increased turnover of tumor cells shed into systemic circulation in DMBA induced HBP carcinogenesis. Our results are in harmony with these lines.
In our present study, atypical expression of glycoconjugates in the tumor tissues of hamsters was confirmed by histological and immunohistochemical. Administration of hesperetin at a dose of 20 mg/kg b.w. to DMBA treated hamsters significantly reduced the levels of glycoconjugates in the circulation and in tumor tissues. Our results suggest that hesperetin significantly reduced the levels of glycoconjugates, cytokeratin and thereby protected the cell surface abnormality, which indicates membrane stabilizing effect by structural integrity indicating its potent anticancer property.
Conclusion
Hesperetin extensively inhibited the abnormalities in cell surface glycoconjugates during DMBA induced oral carcinogenesis and prevented the overexpression of cytokeratin. The protective effect of hesperetin on suppression of glycoprotein synthesis by modulating the activities of the enzymes involved in the glycosylation. Further studies therefore defensible to assess the hesperetin efficacy on the activities of enzymes involved in the process of glycosylation.
Acknowledgements
The financial support from DST-SERB, New Delhi, India in the form of the research project is greatly acknowledged.
Funding
This study was funded by Department of Science and Technology (DST), Science and Engineering Research Board (SERB) India for providing financial support for this research project (DST No: SB/YS/LS-104/2013 dated: 26.11.2013).
Conflict of interest
The authors report no declaration of interest.
Ethical Approval
All the hamsters were maintained in accordance with the guidelines of CPCSEA and the experimental design was permitted by the local institutional animal ethics committee (IAEC), Annamalai University (Proposal No. 1035; Dated 06.18.2013). The animals were kept in compliance with the guide for the care and use of laboratory animals.
References
- 1.Yuan TH, Lian IEB, Tsai KY, Chang TK, Chiang CT, Su CC, Hwang YH. Possible association between nickel and chromium and oral cancer: a case control study in central Taiwan. Sci Total Environ. 2011;409:104–652. doi: 10.1016/j.scitotenv.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 2.Kaur R, Arora S. Interactions of betulinic acid with xenobiotic metabolizing and antioxidative enzymes in DMBA treated sprague dawley female rats. Free Rad Biol Med. 2013;65:131–142. doi: 10.1016/j.freeradbiomed.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Bhavanandan VP, Gowda DC. Introduction to the complexity of cell surface and tissue matrix glycoconjugates. Adv Neurobiol. 2014;9:1–31. doi: 10.1007/978-1-4939-1154-7_1. [DOI] [PubMed] [Google Scholar]
- 4.Chen YT, Ho CL, Chen PK, Chen YL, Chang CF. Anterior gradient 2: a novel sensitive tumor marker for metastatic oral cancer. Cancer Lett. 2013;339:270–278. doi: 10.1016/j.canlet.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Sharmila U, Subramanya U, Prabhu KS. Serum glycoconjugates and ceruloplasmin in cancer of uterine cervix. Ind J Clin Biochem. 2002;17:20–24. doi: 10.1007/BF02867936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep. 2007;18:639–643. [PubMed] [Google Scholar]
- 7.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 8.Leelavinothan P, Kalist S. Beneficial effect of hesperetin on cadmium induced oxidative stress in rats: an in vivo and in vitro study. Eur Rev Med Pharmacol Sci. 2011;15:992–1002. [PubMed] [Google Scholar]
- 9.Cai Y, Luo Q, Sun M, Corke H. Antioxidative activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg AS, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 11.Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao LD, Tsai SY, Hou YC, Hseu YC. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem. 2012;60:522–532. doi: 10.1021/jf2040675. [DOI] [PubMed] [Google Scholar]
- 12.Adams M, Berset C, Kessler M, Hamburger M. Medicinal herbs for the treatment of rheumatic disorders a survey of European herbals from the 16th and 17th century. J Ethnopharmacol. 2009;121:343–359. doi: 10.1016/j.jep.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Yeh CC, Kao SJ, Lin CC, Wang SD, Liu CJ, Kao ST. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007;80:1821–1831. doi: 10.1016/j.lfs.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Gurushankar K, Nazeer SS, Jayasree RS, Krishnakumar N. Evaluation of antitumor activity of hesperetin loaded nanoparticles against DMBA-induced oral carcinogenesis based on tissue autofluorescence spectroscopy and multivariate analysis. J Fluoresc. 2015;25:931–939. doi: 10.1007/s10895-015-1575-4. [DOI] [PubMed] [Google Scholar]
- 15.Niebes P. Determination of enzymes and degradation products of glycosaminoglycans. Clin Chim Acta. 1972;42:399–408. doi: 10.1016/0009-8981(72)90105-2. [DOI] [Google Scholar]
- 16.Wagner WD. More sensitive assay discriminating glactosamine and glucosamine in mixtures. Anal Biochem. 1979;94:394–397. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 17.Warren L. The thiobarbituric acid assay of sialic acid. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 18.Dische L, Shettles LB. Specific colour reactions of methyl pentoses and spectrophotometric micromethod for their determination. J Biol Chem. 1948;175:595–598. [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Krishnamoorthy L, Mahal LK. Glycomic analysis: an array of technologies. ACS Chem Biol. 2009;4:715–732. doi: 10.1021/cb900103n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sindhu G, Manoharan S. Berberine protects cellular integrity during 7,12-dimethyl benz(a)anthracene-induced oral carcinogenesis in golden Syrian hamsters. J Cell Tissue Res. 2010;10:2185–2190. [Google Scholar]
- 22.Drake RR, Schwegler EE, Malik G, Diaz J, Block T, Mehta A, Semmes OJ. Lectin capture strategies combined with mass spectrometry for the discovery of serum glycoprotein biomarkers. Mol Cell Proteomics. 2006;5:1957–1967. doi: 10.1074/mcp.M600176-MCP200. [DOI] [PubMed] [Google Scholar]
- 23.Kaur R, Singh AP, Arora S. Diminution of hepatic response to 7,12 dimethylbenz(a)anthracene by ethyl acetate fraction of Acacia catechu willd. through modulation of xenobiotic and antioxidative enzymes in rats. PLoS ONE. 2014;9:e90083. doi: 10.1371/journal.pone.0090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo CB, Li YA, Gao Y. Immunohistochemical staining with cytokeratin combining semi-serial sections for detection of cervical lymph node metastases of oral squamous cell carcinoma. Auris Nasus Larynx. 2007;34:347–351. doi: 10.1016/j.anl.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Rajkamal G, Suresh K, Sugunadevi G, Vijayaanand MA, Rajalingam K. Evaluation of chemopreventive effects of Thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMB Rep. 2010;43:664. doi: 10.5483/BMBRep.2010.43.10.664. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan P, Sabitha KE, Shyamaladevi CS. Modulatory efficacy of Green tea polyphenols on glycoconjugates and immunological markers in 4-nitroquinoline 1-oxide-induced oral carcinogenesis–a therapeutic approach. Chem Biol Interact. 2006;162:149–156. doi: 10.1016/j.cbi.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Bose KS, Gokhale PV, Dwivedi S, Singh M. Quantitative evaluation and correlation of serum glycoconjugates: protein bound hexoses, sialic acid and fucose in leukoplakia, oral sub mucous fibrosis and oral cancer. J Nat Sci Biol Med. 2013;4:122–125. doi: 10.4103/0976-9668.107275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramkumar S, Sakac D, Binnington B, Branch DR, Lingwood CA. Induction of HIV resistance: cell susceptibility to infection is an inverse function of globotriaosyl ceramide levels. Glycobiology. 2009;19:76–82. doi: 10.1093/glycob/cwn106. [DOI] [PubMed] [Google Scholar]
- 29.Taqi SH. Clinical evaluation of total and lipid bound sialic acid levels in oral precancer and oral cancer. Indian J Med Paediatr Oncol. 2012;33:36–41. doi: 10.4103/0971-5851.96967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinothkumar V, Manoharan S, Planimuthu D, Rajasekaran D, Srinivasan R, Afaqwani S. Geraniol protects cell surface glycoconjugates during 7,12 dimethylbenz(a)anthracene induced oral carcinogenesis. J Cell Tissue Res. 2011;11:2759–2764. [Google Scholar]