Abstract
This study evaluated contribution of minor compounds naturally present in peppermint (Mentha piperita) to the iron-catalyzed lipid oxidation of oil-in-water emulsion. Emulsions consisted of tocopherol-stripped soybean oil and pH 4.0 citrate buffer (4:6, w/w) with iron. Minor compounds included α-tocopherol, rosmarinic acid, caffeic acid, β-carotene, and chlorophyll b at natural concentration in 400 ppm of the peppermint extract. The emulsions were oxidized in the dark, and headspace oxygen contents, hydroperoxide contents, and p-anisidine values were determined. Addition of phenolic compounds decreased headspace oxygen consumption and hydroperoxide and p-anisidine values of emulsions, however, β-carotene or chlorophyll b tended to increase them. The results suggest that tocopherols at low concentration were the most important to reduce lipid oxidation of emulsions via radical scavenging, followed by high contents of polyphenols via radical scavenging and iron-chelation. Carotenoids and chlorophylls should be precisely controlled even in the dark, possibly due to their oxidation products.
Keywords: Peppermint extract, Minor compounds, Iron-catalyzed lipid oxidation, Oil-in-water emulsion
Introduction
Peppermint (Mentha piperita L.) extract was reported to reduce lipid oxidation of oil-in-water emulsions, most of which was explained with polyphenols. Phenolic acid, especially rosmarinic acid (> 30%) is a main group among polyphenols in the peppermnit extract (Kim and Choe, 2018). It has been reported that phenolic compounds including polyphenols and tocopherols exhibit antioxidant or prooxidant activity both in foods (Fukumoto and Mazza, 2000) and in vivo and vitro (Banerjee et al., 2008; Lambert and Elias, 2010). Phenolic compounds act as antioxidants by radical scavenging, metal chelation, and physical restriction of the metal ion for participation in Fenton-type reactions (Cheng et al., 2003). However, they can be oxidized to produce semiquinone and more reactive superoxide anion radicals (Mochizuki et al., 2002) which increase lipid oxidation. Transition metal ions such as Fe(III) or Cu(II) increase the prooxidative activity of phenolic compounds by producing more prooxidative Fe(II) or Cu(I) by reduction (Simić et al., 2007).
Peppermint also contains chlorophylls and carotenoids (Song and Choe, 2017) which have radical scavenging activity in the dark. However, carotenoids can accelerate the lipid oxidation depending on their concentration and chlorophylls also show prooxidant activity under light by producing reactive singlet oxygen (Choe and Min, 2006). Recently tocopherols were detected in the peppermint extract (Song and Choe, 2017), and they act as both antioxidants and prooxidants depending on the concentration (Choe and Min, 2006). Because oxidative stability of emulsions is an overall result of anti- or pro-oxidant activities of minor compounds, the antioxidant activity of peppermint extract in the emulsion should be evaluated with consideration of the kinds and natural concentration of minor compounds present in the extract. This study determined the contribution of minor compounds naturally present in the peppermint extract to the lipid oxidation of oil-in-water emulsion in the presence of iron in the dark.
Materials and methods
Materials and reagents
Refined, bleached, and deodorized (RBD) soybean oil was a product of Samyang Corp. (Seoul, Korea), and peppermint (M. piperita) was purchased from the Hwanamnongsan (Greenfarm) (Seoul, Korea) in fresh form which was then freeze-dried for 24 h with a TFD5505 freeze dryer (Ilshinbiobase, Dongducheon, Korea) at − 50 °C and 5 mtorr. The egg yolk lecithin was a product of Goshen Biotech (Namyangju, Korea), and n-hexane, water, and methanol in HPLC grade were purchased from Samchun Chemical Co. (Seoul, Korea). Acid washed silicic acid (200–400 mesh), aluminum oxide (type WN-3, neutral), Folin–Ciocalteu’s phenol reagent, cumene hydroperoxide (CuOOH), p-anisidine, xanthan gum, ammonium thiocyanate, α-, γ-, and δ-tocopherol, rosmarinic acid, caffeic acid, β-carotene, lutein, and chlorophyll a and b were purchased from the Sigma-Aldrich Co. (Saint Louis, MO, USA). Propan-2-ol and ferrous sulfate were purchased from the Mallinckrodt Baker Co. (Phillipsburg, NJ, USA) and Junsei Co. (Tokyo, Japan), respectively. All other chemicals were of analytical grade.
Preparation of peppermint extract
Freeze-dried peppermint was ground in an Essence HR 2084 blender (Philips, Amsterdam, Netherlands), and then mixed with 75% ethanol (1:10, w/v) at 25 °C and 120 rpm for 12 h. The extract was obtained after filtration through a Whatmann #42 paper (GE Healthcare Life Sciences, Little Chalfont, UK) and solvent was removed by evaporation at 65 °C using a rotary evaporator (N–N series; Eyela, Tokyo, Japan).
Preparation of emulsions and oxidation
The emulsion consisted mainly of tocopherol-stripped soybean oil and citrate buffer solution at pH 4.0 (4:6, w/w), with xanthan gum (3.5 mg/kg) and egg yolk lecithin (3.5 mg/kg) according to Kim and Choe (2016). Tocopherol-stripped oil was obtained by passing the RBD soybean oil through a glass column (30 × 2.5 cm) packed with silicic acid, aluminum oxide, and silicic acid (5:35:5, w/w/w) in series (Kim and Choe, 2016). Soybean oil and egg yolk lecithin were slowly added to the aqueous solution containing FeSO4 (5 mg/kg), and then α-tocopherol (0.56 mg/kg), rosmarinic acid (68.4 mg/kg), caffeic acid (68.4 mg/kg), β-carotene (0.22 mg/kg), or chlorophyll b (1.78 mg/kg) was selectively added. The solution with all ingredients was then homogenized using an Ultra-Turrax T25 homogenizer (IKA Instruments, Staufen, Germany) at 10,000 rpm for 6 min. The emulsions transferred into 20 mL serum bottles were tightly capped with Teflon-coated septa (Cronus, Glocester, England) and aluminum caps. All samples were oxidized in an LBI-250 incubator (Daihan Labtech Co., Seoul, Korea) at 25 °C for 4 days in the dark.
Analysis of lipid oxidation of emulsions
Lipid oxidation of emulsions was evaluated based on the headspace oxygen content by gas chromatography, hydroperoxide values by the ferric thiocyanate method, and p-anisidine values. A YL 6100 gas chromatograph (Younglin Instrument Co., Ltd., Anyang, Korea) was used for oxygen analysis with an autosampler, a thermal conductivity detector, and a stainless steel column packed with 80/100 mesh molecular sieve 13X (1.83 m × 0.32 cm; Alltech, Deerfield, IL, USA). The flow rate of nitrogen was 20 mL/min. The temperatures of the oven, injector, and detector were 35, 100, and 140 °C, respectively. A factor of 9.35 was applied to convert oxygen peak areas in the chromatograms to μmol of oxygen/mL of headspace gas (Choe and Choe, 2016). The hydroperoxide contents of emulsions were determined spectrophotometrically after extracting the organic phase with mixture of 2,2,4-trimethylpentane and propan-2-ol (3:1, v/v), followed by serial addition of methanol and trichloromethane mixture (2:1, v/v), 3.94 M ammonium thiocyanate solution, and a mixture of 0.132 M BaCl2 and 0.144 M FeSO4 solution (1:1, v/v) (Kim and Choe, 2016). The absorbance was read at 510 nm using an HP8453 UV–visible spectrophotometer (UV-2700, Shimadzu Corp., Kyoto, Japan) and the hydroperoxide content was expressed as CuOOH using a calibration curve (r2 = 0.9996). The p-anisidine value was determined spectrophotometrically (350 nm) using a UV–visible spectrophotometer (UV-2700, Shimadzu Corp.) according to the AOCS method Cd 18-90 (AOCS, 2006).
Phenolic compound and pigment determination of peppermint extract and emulsions
Tocopherol content of the peppermint extract was determined by the HPLC (Song and Choe, 2017). The extract dissolved in n-hexane was filtered through a hydrophobic polytetrafluoroethylene membrane filter (0.2 μm, Toyo Roshi Kaisha, Ltd., Utsunomiya, Japan) and injected into a YL 9100 HPLC (Younglin Instrument Co., Ltd.) equipped with an autosampler, μ-porasil™ column (3.9 mm × 330 mm, 10 μm size; Waters Co., Milford, USA), and fluorescence detector (excitation 290 nm, emission 330 nm). An eluting solvent was a mixture of propan-2-ol and n-hexane (0.2:99.8, v/v) at 2.0 mL/min. Quantification was done with a calibration curve of standard α-, γ-, and δ-tocopherol (r2 > 0.998). Total contents of polyphenols of the peppermnint extract were determined spectrophotometrically at 725 nm using a UV–visible spectrophotometer (UV-2700, Shimadzu Corp.) according to the Folin–Ciocalteu method (Kim and Choe, 2016), and expressed as a caffeic acid or rosmarinic acid equivalent (r2 > 0.999). Carotenoids and chlorophylls of the peppermint extract were analyzed using HPLC (Kim and Choe, 2017). The extract was saponified following the AOAC method 970.64 (AOAC, 2005) only for carotenoid determination. The instrument was a YL 9100 HPLC (Younglin Instrument Co., Ltd.) equipped with a symmetry C18 column (5.0 μm, 4.6 × 300 mm, Waters) and UV–Vis detector (438 nm) or a μ-porasil column (300 × 3.9 mm, 10 μm i.d., Waters) and UV–Vis detector (436 nm) for chlorophyll and carotenoid determination, respectively. The mobile phase for chlorophyll and carotenoid determination was ethyl ethanoate:methanol:water (50:37.5:12.5, v/v/v) at 1.5 mL/min and propan-2-ol: n-hexane (3:97, v/v) at 1.0 mL/min, respectively. Carotenoids and chlorophylls were identified by comparing the retention times with standard lutein, β-carotene, and chlorophyll a and b, and quantified using respective calibration curves (r2 > 0.998).
Polyphenol contents of emulsions were determined by the same method as described above after obtaining aqueous phase by centrifugation (Avanti J; Beckman Co.; 4 °C and 11,872×g) for 20 min of the mixture of emulsion dissolved in n-hexane and 60% methanol in water (10:6, v/v). For determination of tocopherol and pigment contents of emulsions, the emulsions were frozen for 48 h and then thawed for 24 h, followed by centrifugation (Avanti. J, Beckman, Fullerton, CA, USA) at 4 °C and 12,935×g for 20 min for phase separation (Song and Choe, 2017). Organic phase was taken and tocopherol, carotenoid, and chlorophyll contents were determined with the same methods described above.
Statistical analysis
All samples were prepared in duplicates, and each sample was measured twice. Data were presented with means and standard deviations and statistically analyzed using SAS/PC (SAS 9.2, SAS Institute Inc., Cary, NC, USA) including Duncan’s multiple range test at a significance level of 5%.
Results and discussion
Phenolic compound and pigment contents of peppermint extract
The peppermint extract contained tocopherols totally at 1323.8 ± 3.38 mg/kg with α-tocopherol (1087.8 ± 4.20 mg/kg) and γ-tocopherol (236.0 ± 0.82 mg/kg), and polyphenols totally at 168.98 ± 3.44 g/kg. β-Carotene (509.7 ± 20.37 mg/kg) was major carotenoid in the extract with less amount of lutein (10.2 ± 0.00 mg/kg), and chlorophyll content of the extract was totally 4403.4 ± 6.67 mg/kg with chlorophyll a at 1616.2 ± 0.49 mg/kg and chlorophyll b at 2787.2 ± 7.17 mg/kg. Rosmarinic and caffeic acids were reported as dorminant polyphenol in the peppermint (Guedon and Pasquier, 1994; Hadjmohammadi et al., 2013; Kim and Choe, 2016). Based on the phenolic compound and pigment concentrations of the peppermint extract determined, addition level of each standard minor compound in 1 kg of emulsions was determined to be 0.56, 68.4, 68.4, 0.22, and 1.78 mg for α-tocopherol, rosmarinic acid, caffeic acid, β-carotene, and chlorophyll b, respectively, assuming 400 mg/kg as an addition level of the peppermint extract to the emulsion. The lipid oxidative stability of soybean oil-in-water emulsion (4:6, w/w) was significantly improved by the addition of peppermint extract at 400 mg/kg in our previous study (Kim and Choe, 2016).
Lipid oxidation of emulsions
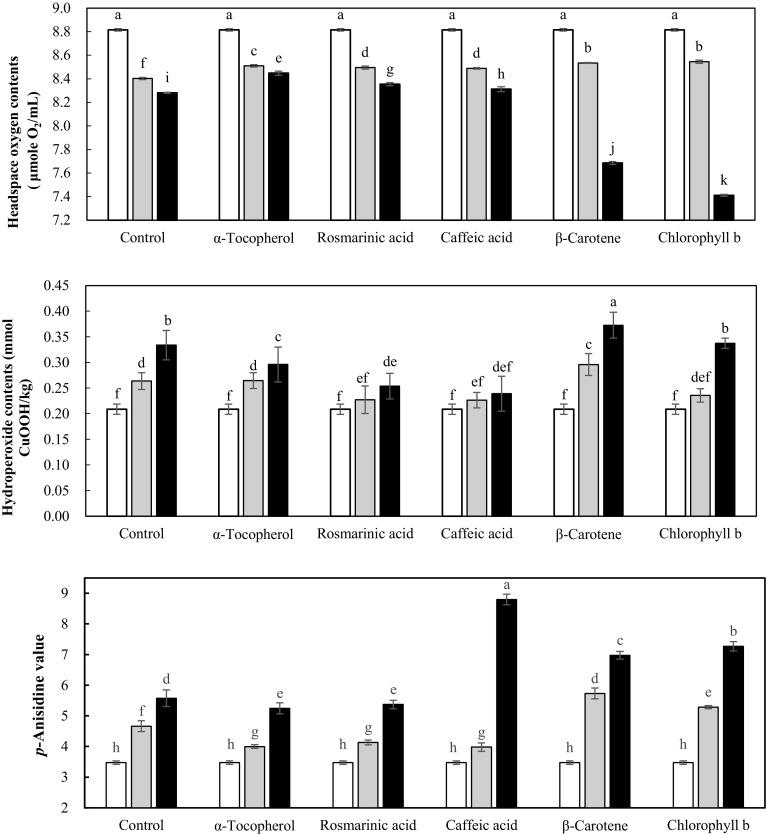
Degree of lipid oxidation at 25 °C in the dark for 4 days, based on the headspace oxygen contents, hydroperoxide contents, and p-anisidine values, of the soybean oil-in-water (4:6, w/w) emulsion with added standard phenolic compounds and pigments at natural concentration present in the peppermint extract in the presence of iron (5 mg/kg) is shown in Fig. 1. The headspace oxygen content of emulsions was 8.82 μmol/mL before oxidation, but significantly (p < 0.05) decreased with oxidation time due to oxygen consumption for the lipid oxidation. After a 2 day oxidation, headspace oxygen content of the emulsion with added α-tocopherol, rosmarinic acid, caffeic acid, β-carotene, or chlorophyll b was significantly (p < 0.05) higher (8.51, 8.49, 8.49, 8.53, and 8.54 μmol/mL, respectively) compared to that of the control emulsion without addition of any minor compounds present in the peppermint extract (8.40 μmol/mL). This indicates that addition of these compounds reduced headspace oxygen consumption of the emulsion. Addition of phenolic compounds reduced headspace oxygen consumption after 4 days, too. The effect to decrease oxygen consumption in the headspace of the emulsion was significantly higher by α-tocopherol than by rosmarinic or caffeic acid (p < 0.05) although the addition level was opposite. The oxygen content of the emulsion with added β-carotene (7.69 μmol/mL) or chlorophyll b (7.41 μmol/mL) after 4 days, however, was significantly (p < 0.05) lower than that of the control emulsion (8.28 μmol/mL), which indicates increased oxygen consumption in the headspace by chlorophyll b or β-carotene. The hydroperoxide contents of emulsions were significantly (p < 0.05) increased during 4 day oxidation due to hydroperoxide production. The emulsion with added α-tocopherol, rosmarinic acid, or caffeic acid showed significantly (p < 0.05) reduced hydroperoxide contents than the control emulsion, however, addition of β-carotene resulted in significantly (p < 0.05) higher hydroperoxide production (0.30 and 0.37 mmol/kg after 2 and 4 days, respectively) than the control emulsion (0.26 and 0.33 mmol/kg after 2 and 4 days, respectively). There was no significant effect (p > 0.05) of chlorophyll b (0.24 and 0.34 mmol/kg after 2 and 4 days, respectively) on the hydroperoxide contents of emulsions during oxidation. The p-anisidine values also increased with oxidation time due to hydroperoxide decomposition into aldehydes. Addition of α-tocopherol (4.00 and 5.25 after 2 and 4 days, respectively) or rosmarinic acid (4.13 and 5.37 after 2 and 4 days, respectively) significantly (p < 0.05) reduced the p-anisidine values compared to that of the control emulsion (4.66 and 5.57 after 2 and 4 days, respectively). However, the emulsions with added β-carotene (5.73 and 6.97 after 2 and 4 days, respectively) or chlorophyll b (5.29 and 7.27 after 2 and 4 days, respectively) showed significantly (p < 0.05) higher p-anisidine values than the control emulsion during oxidation. Results on the headspace oxygen contents, hydroperoxide contents, and p-anisidine values indicated that α-tocopherol, rosmarinic acid, and caffeic acid at natural concentration present in the peppermint extract acted as antioxidant in the iron-catalyzed lipid oxidation of the soybean oil-in-water emulsion, but β-carotene and chlorophyll b tended to increase the lipid oxidation in spite of the dark condition. Chlorophylls and β-carotene have been known to decrease the lipid oxidation in the dark via radical scavenging, however, their oxidation products can increase the lipid oxidation (Choe and Min, 2009). When peroxy radical is added to β-carotene during oxidation, carotene peroxy radicals are produced (Burton and Ingold, 1984), which in turn reacts with atmospheric oxygen and then with lipid molecules to propagate the chain reaction of lipid oxidation (Iannone et al., 1998). The oxidation of chlorophylls produces epoxides or diperoxides which can accelerate the lipid oxidation (Hahm, 1988). The overall results on the lipid oxidation strongly suggest that contribution to the reduced lipid oxidation of soybean oil-in-water (4:6, w/w) emulsion was the highest by α-tocopherol despite its very low concentration in the emulsion (0.56 mg/kg), followed by high concentration of rosmarinic and caffeic (68.4 mg/kg) acids among minor compounds naturally present in the peppermint. Chlorophyll b (1.78 mg/kg) and β-carotene (0.22 mg/kg) should be also controlled for oxidation-stable soybean oil emulsions even in the absence of light and low concentrations due to their prooxidant activity.
Fig. 1.
Effect of minor compound addition on the iron-catalyzed lipid oxidation of soybean oil-in-water (40:60, w/w) emulsion at 25 °C in the dark (white square: 0 day; grey square: after 2 days; black square: after 4 days). Different letters on the line represent significantly different values by Duncan’s multiple range test at 5%
Minor compound contents of emulsions during oxidation
Contents of phenolic compounds and pigments of soybean oil-in-water (4:6, w/w) emulsions during 4 day oxidation are shown in Table 1. Tocopherols were not detected in the emulsions throughout 4 day oxidation, possibly due to fast degradation and very low addition level. α-Tocopherol is an excellent antioxidant to scavenge peroxy radicals by rapidly donating phenolic hydrogens (Choe and Min, 2009), and resulting phenolic radical can react with another peroxy radicals to form phenolic peroxy species adducts that undergo the degradation reactions (Reische et al., 2002). Although α-tocopherol at 20–60 μg/mL showed 58% inhibition of the Fe2+-ferrozine complex formation (Gulcin et al., 2003), its radical scavenging activity is much higher (5 times) than that of iron-chelation (Yüksek et al., 2008), suggesting its possible fast degradation. α-Tocopherol showed higher radical and hydrogen peroxide scavenging activity than polyphenols derived from methanolic extract of Ichnocarpus frutescens in the lipid oxidation of rat brain homogenate (Kumarappan et al., 2012). Rosmarinic acid content was significantly (p < 0.05) decreased to 30.52 and 29.28 mg/kg after 2 and 4 day oxidation of the emulsion, respectively, indicating their degradation. When polyphenols donate phenolic hydrogens to the peroxy radicals, they are degraded to quinones (Choe and Min, 2009). Caffeic acid was also degraded during the emulsion oxidation, and its degradation was higher (65.8% retention after 4 days) than rosmarinic acid (88.0% retention after 4 days). Degradation of polyphenols was suggested to be related to their role as radical scavengers (Kim and Choe, 2017), and the result thus suggests higher radical scavenging activity of caffeic acid than that of rosmarinic acid. The ABTS+ radical scavenging activity of caffeic acid was higher than that of rosmarinic acid, but vice versa for superoxide anion radical scavenging (Kovacheva et al., 2006). Adomako-Bonsu et al. (2017) reported no significant difference (p > 0.05) in the DPPH radical scavenging activity between these two phenolic acids. Polyphenols in the peppermint extract were suggested to participate less in radical scavenging than in iron chelation in the iron-catalyzed lipid oxidation of oil-in-water emulsions (Kim and Choe, 2018), which supports our result of lower degradation of polyphenols than α-tocopherol.
Table 1.
Content (mg/kg) of minor compounds in the soybean oil-in-water (40:60, w/w) emulsion containing iron (5 mg/kg) during oxidation at 25 °C in the dark
| Oxidation time (day) | α-Tocopherol | Rosmarinic acid | Caffeic acid | β-Carotene | Chlorophyll b |
|---|---|---|---|---|---|
| 0 | n.d.1) | 33.29 ± 2.487a2) (100)3) | 31.61 ± 2.173ab (100) | 0.162 ± 0.004a (100) | 0.862 ± 0.029a (100) |
| 2 | n.d. | 30.52 ± 1.931ab (91.7) | 22.93 ± 1.901c (72.5) | 0.168 ± 0.010a (103.7) | 0.761 ± 0.027a (88.3) |
| 4 | n.d. | 29.28 ± 2.503b (88.0) | 20.80 ± 0.983c (65.8) | 0.155 ± 0.004a (95.7) | 0.291 ± 0.368b (33.8) |
1) Not detected
2) Different letters represent significantly different values in the same column by Duncan’s multiple range test at 5%
3) Retention percentage based on the initial content before oxidation (%)
There was no significant change (p > 0.05) in β-carotene content during 4 day oxidation of the soybean oil-in-water (4:6, w/w) emulsions. This might be due to little radical scavenging activity of β-carotene. β-Carotene is less reactive toward peroxy radical than α-tocopherol (Tsuchihashi et al., 1995), which might have resulted in little degradation of β-carotene. Increased lipid oxidation of the emulsion with added β-carotene in this study thus might be due to its little peroxy radical scavenging. Although a possibility of astaxanthin to form a complex with Cu+2 was suggested (Hernández-Marin et al., 2012), iron chelating effect of β-carotene was reported to be very limited (de Arruda and Canniatti-Brazaca, 2011). Chlorophyll b content of the emulsion was 0.862 mg/kg before oxidation, and significantly (p < 0.05) decreased to 0.761 and 0.291 mg/kg after 2 and 4 days, respectively, due to its degradation. Chlorophylls act as antioxidant in the dark via scavenging of hydroxy and peroxy radicals and iron-chelation (Hsu et al., 2013). High lipid oxidation after 4 days in the emulsion with added chlorophyll b could be due to rapid degradation of chlorophyll b in the emulsion. Degradation of chlorophyll b in the dark was suggested to be through radical mechanism (Lee et al., 2014). When the phytyl chain of chlorophylls is autoxidized, peroxy radicals are added to the double bond and resulting tertiary radical can produce epoxides or diperoxides (Rontani and Aubert, 1994; Rontani and Aubert, 2005) which might be prooxidant. Formation of iron-chlorophyll complex is possible, but at less degree than copper-chlorophyll complex (Zvezdanovic and Markovic, 2009).
In conclusion, α-tocopherol at very low concentration (< 1 mg/kg) among minor compounds naturally present in the peppermint extract was the most important to reduce lipid oxidation of the soybean oil-in-water (4:6, w/w) emulsion mostly via radical scavenging. Rosmarinic and caffeic acids also contributed to the decreased lipid oxidation via radical scavenging and iron-chelation mainly due to high concentration. Chlorophyll b and β-carotene tended to increase the lipid oxidation. The results strongly suggest that tocopherols present at very low concentration in the peppermint are the most important antioxidant, and high concentration is the main reason for polyphenols’ antioxidant activity. Chlorophylls and carotenoids present in the peppermint should be controlled for oxidation-stable oil-in-water emulsion even in the absence of light and at low concentration, possibly due to their oxidation products.
Acknowledgements
This research was supported by the Inha University (55832-01), for which the authors are grateful.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Adomako-Bonsu AG, Chan SLF, Pratten M, Fry JR. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- AOAC . Official Method of Analysis of AOAC International: Method 970.64. 18. Gaithersburg, MD: Association of Official Analytical Chemists; 2005. [Google Scholar]
- AOCS . Official Methods and Recommended Practices of the American Oil Chemists’ Society: Method Cd 18-90. 4. Champaign, IL: AOCS Press; 2006. [Google Scholar]
- Banerjee A, Kunwar A, Mishra B, Priyadarsini KI. Concentration dependent antioxidant/pro-oxidant activity of curcumin, studies from AAPH induced hemolysis of RBCs. Chem. Biol. Interact. 2008;174:134–139. doi: 10.1016/j.cbi.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Burton GW, Ingold KU. β-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Li Y, Chang W. Kinetic deoxyribose degradation assay and its application in assessing the antioxidant activities of phenolic compounds in a Fenton- type reaction system. Anal. Chim. Acta. 2003;478:129–137. doi: 10.1016/S0003-2670(02)01435-6. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Comp. Rev. Food Sci. Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Choe E, Min DB. Mechanisms of antioxidants in the oxidation of foods. Comp. Rev. Food Sci. Food Saf. 2009;8:345–358. doi: 10.1111/j.1541-4337.2009.00085.x. [DOI] [Google Scholar]
- Choe J, Choe E. Effect of soy-derived phospholipid on the autoxidation of canola oil in a water/oil emulsion. J. Am. Oil Chem. Soc. 2016;93:1085–1094. doi: 10.1007/s11746-016-2855-0. [DOI] [Google Scholar]
- de Arruda GR, Canniatti-Brazaca SG. Iron availability in the presence of β-carotene in different mixtures. Ciênc. Tecnol. Aliment. Campinas. 2011;31:327–333. doi: 10.1590/S0101-20612011000200008. [DOI] [Google Scholar]
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Guedon DJ, Pasquier BP. Analysis and distribution of flavonoid glycosides and rosmarinic acid in 40 Mentha piperita clones. J. Agric. Food Chem. 1994;42:679–684. doi: 10.1021/jf00039a015. [DOI] [Google Scholar]
- Gulcin I, Buyukokuroglu ME, Kufrevioglu OI. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003;34:278–281. doi: 10.1034/j.1600-079X.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Hadjmohammadi M, Karimiyan H, Sharifi V. Hollow fibre-based liquid phase microextraction combined with high-performance liquid chromatography for the analysis of flavonoids in Echinophora platyloba DC and Mentha piperita. Food Chem. 2013;141:731–735. doi: 10.1016/j.foodchem.2013.02.083. [DOI] [PubMed] [Google Scholar]
- Hahm TS. Effects of initial peroxide contents on the oxidative stability of soybean oil to prevent environmental pollution. J. Environ. Research. 1988;1:112–120. [Google Scholar]
- Hernández-Marin E, Barbosa A, Martínez A. The metal cation chelating capacity of astaxanthin. Does this have any influence on antiradical activity? Molecules. 2012;17:1039–1054. doi: 10.3390/molecules17011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Chao P-Y, Hu S-P, Yang C-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr. Sci. 2013;4:1–8. [Google Scholar]
- Iannone A, Rota C, Bergamini S, Tomasi A, Canfield LM. Antioxidant activity of carotenoids: an electron-spin resonance study on β-carotene and lutein interaction with free radicals generated in a chemical system. J. Biochem. Mol. Toxicol. 1998;12:299–304. doi: 10.1002/(SICI)1099-0461(1998)12:5<299::AID-JBT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kim J, Choe E. Effect of the pH on the lipid oxidation and polyphenols of soybean oil-in-water emulsion with added peppermint (Mentha piperita) extract in the presence and absence of iron. Food Sci. Biotechnol. 27: In press (2018) [DOI] [PMC free article] [PubMed]
- Kim J, Choe E. Effects of selected herb extracts on iron-catalyzed lipid oxidation in soybean oil-in-water emulsion. Food Sci. Biotechnol. 2016;25:1017–1022. doi: 10.1007/s10068-016-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Choe E. Improvement of the lipid oxidative stability of soybean oil-in water emulsion by addition of daraesoon (shoot of Actinidia arguta) and samnamul (shoot of Aruncus dioicus) extract. Food Sci. Biotechnol. 2017;26:113–119. doi: 10.1007/s10068-017-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacheva E, Georgiev M, Pashova S, Angelova M, Ilieva M. Radical quenching by rosmarinic acid from Lavandula vera MM cell culture. Z. Naturforsch C. 2006;61:517–520. doi: 10.1515/znc-2006-7-808. [DOI] [PubMed] [Google Scholar]
- Kumarappan CT, Thilagam E, Mandal SC. Antioxidant activity of polyphenolic extracts of Ichnocarpus frutescens. Saudi J. Biol. Sci. 2012;19:349–355. doi: 10.1016/j.sjbs.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch. Biochem. Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Ahn H, Choe E. Effects of light and lipids on chlorophyll degradation. Food Sci. Biotechnol. 2014;23:1061–1065. doi: 10.1007/s10068-014-0145-x. [DOI] [Google Scholar]
- Mochizuki M, Yamazaki S, Kano K, Ikeda T. Kinetic analysis and mechanistic aspects of autoxidation of catehins. Biochim. Biophys. Acta. 2002;1569:35–44. doi: 10.1016/S0304-4165(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Reische David, Lillard Dorris, Eitenmiller Ronald. Food Science and Technology. 2002. Antioxidants. [Google Scholar]
- Rontani J-F, Aubert C. Characterization of isomeric allylic diols resulting from chlorophyll phytyl side-chain photo- and autoxidation by electron ionization gas chromatography/mass spectrometry. Rapid Commun. Mass Sp. 2005;19:637–646. doi: 10.1002/rcm.1835. [DOI] [PubMed] [Google Scholar]
- Rontani J-F, Aubert C. Effect of oxy-free radicals upon the phytyl chain during chlorophyll-a photodegradation. J. Photochem. Photobiol. A. 1994;79:167–172. doi: 10.1016/1010-6030(93)03762-6. [DOI] [Google Scholar]
- Simić A, Manojlović D, Šegan D, Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12:2327–2340. doi: 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, Choe E. Effect of sannamul and herb extract addition on the photooxidation of soybean oil emulsion. Korean J. Food Cook. Sci. 2017;33:275–284. doi: 10.9724/kfcs.2017.33.3.275. [DOI] [Google Scholar]
- Tsuchihashi H, Kigoshi M, Iwatsuki M, Niki E. Action of beta-carotene as an antioxidant against lipid peroxidation. Arch. Biochem. Biophys. 1995;323:137–147. doi: 10.1006/abbi.1995.0019. [DOI] [PubMed] [Google Scholar]
- Yüksek H, Alkan M, Cakmak I, Ocak Z, Bahçeci Ş, Calapoğlu M, Elmastaş M, Kolomuç A, Aksu H. Preparation, GIAO NMR calculations and acidic properties of some novel 4,5-dihydro-1H-1,2,4-triazol-5-one derivatives with their antioxidant activities. Int. J. Mol. Sci. 2008;9:12–32. doi: 10.3390/ijms9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvezdanovic J, Markovic D. Copper, iron, and zinc interactions with chlorophyll in extracts of photosynthetic pigments studied by Vis spectroscopy. Russ. J. Phys. Chem. A. 2009;83:1542–1546. doi: 10.1134/S0036024409090222. [DOI] [Google Scholar]