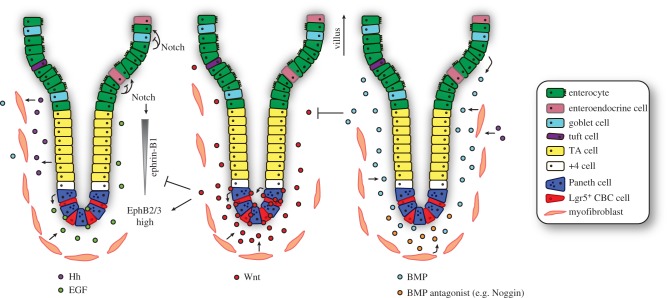
Figure 3.
Interconnectivity of signalling pathways governs crypt–villus homeostasis. Intestinal homeostasis and cell fate determination are maintained by interconnectivity of key signalling pathways between epithelial and mesenchymal cells. Wnt ligands (centre) secreted from the Paneth cells and intestinal subepithelial myofibrobasts act predominantly at the base of the crypt to maintain stem cell function and TA cell proliferation. High levels of Wnt signalling induce the expression of EphB2 (ISCs) and EphB3 (Paneth cells), and concurrently repress transcription of the repulsive ligand ephrin-B1. The decline in Wnt signals along the axis results in increased ephrin-B1 levels and proper positioning of the cells. EGF signalling (left) is required for proliferation and maintenance of ISCs and is produced in the ISC niche by the surrounding Paneth cells and subepithelial mesenchyme. Notch signalling regulates cell fate through cell-to-cell contact in the crypt (here for simplicity only visualized on the top left). Notch signalling controls the binary cell fate decision between the secretory and absorptive lineages. Hedgehog (Hh), expressed by epithelial cells in the upper part of the crypt, acts upon and maintains the myofibroblasts. This has a secondary effect on the epithelium through promotion of BMP ligand expression. BMP ligands are predominantly produced by the mesenchymal cells and partly by epithelial villus cells. Mesenchyme-to-epithelium BMP signalling promotes differentiation of progenitor cells while restraining cell proliferation. BMP signalling within the crypt stem cell niche is therefore carefully regulated by BMP antagonists expressed by the mesenchyme surrounding the crypt. Finally, BMP represses Wnt signalling and is expressed in an opposing gradient along the crypt–villus axis.