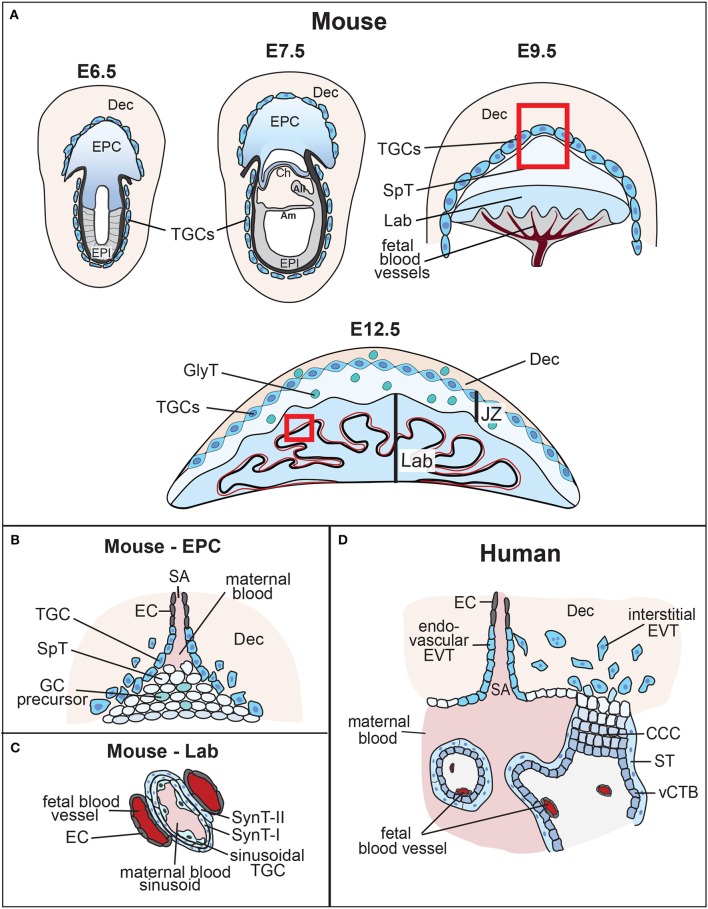
Figure 1.
Overview of mouse placental development and similarities to human placenta. (A) Following implantation, the blastocyst's mural trophectoderm differentiates into trophoblast giant cells (TGCs), while trophectodermal cells that overlie the inner cell mass form the extra-embryonic ectoderm (ExE) and the ectoplacental cone (EPC). The future embryo originates from the blastocyst's inner cell mass that differentiates into the epiblast (EPI). The conceptus is embedded into the maternal decidua (Dec) which differentiates from the uterine endometrium. With gastrulation, the chorion (Ch) consisting of a trophoblast and an extra-embryonic mesodermal cell layer, the amnion (Am) and the allantois (All) are formed. Cells at the margins of the of the EPC differentiate into invasive, secondary TGCs, that remodel the maternal vasculature [highlighted by the inset, see (B)]. The allantois grows out and attaches to the chorion (chorio-allantoic fusion) around E8.5, a critical process for developmental progression. At E9.5, allantoic blood vessels start to invaginate into the chorionic ectoderm to initiate formation of the placental labyrinth (Lab). Trophoblast cells overlying this layer differentiate into future spongiotrophoblast (SpT) and glycogen cells (GCs). The mature mouse placenta is established around mid-gestation (~E10.5) and continues to grow in size and complexity. It consists of three main layers: the labyrinth (Lab), the junctional zone (JZ) made up of SpT, GCs and TGCs, and the maternal decidua (Dec). The labyrinth is the main site of nutrient and gas exchange [highlighted by the inset, see (C)]. Black bar indicates thickness of the labyrinth zone. (B) Magnified view of the tip of the EPC where invasive TGCs remodel maternal spiral arteries (SA) by eroding their smooth muscle lining and displacing their endothelial cell (EC) layer. (C) Close-up view of the interhaemal barrier, consisting (from maternal to fetal side) of a discontinuous layer of sinusoidal TGCs, two layers of syncytiotrophoblast (SynT-I and -II), and the endothelial cell layer of the fetal blood vessels. (D) Despite significant morphological differences, comparison with the human placenta reveals structural and/or functional similarities. Human placental villi are made up of a mesenchymal core that contains the fetal blood vessels, a layer of villous cytotrophoblast (vCTB) and one overlying layer of syncytiotrophoblast (ST) that is directly exposed to maternal blood. Thus, the haemochorial organization and the exchange barrier are similarly organized between mouse and human placentas. vCTBs are perhaps most analogous to the chorionic ectoderm in mice. In anchoring villi, cytotrophoblast cells form cytotrophoblast cell columns (CCCs) that invade into the maternal decidua (Dec). Cells at the base of the CCCs proliferate, pushing cells along the column where they progressively differentiate into invasive extravillous trophoblast (EVT). Trophoblast invasion occurs along two routes, interstitially into the decidual stroma, and along an endovascular route to replace the endothelial cell (EC) lining of maternal spiral arteries (SA). This spiral artery remodeling process is instrumental for healthy pregnancy progression, and is equally shared with the mouse where these processes occur between ~E7.5 and E10.5 as shown.