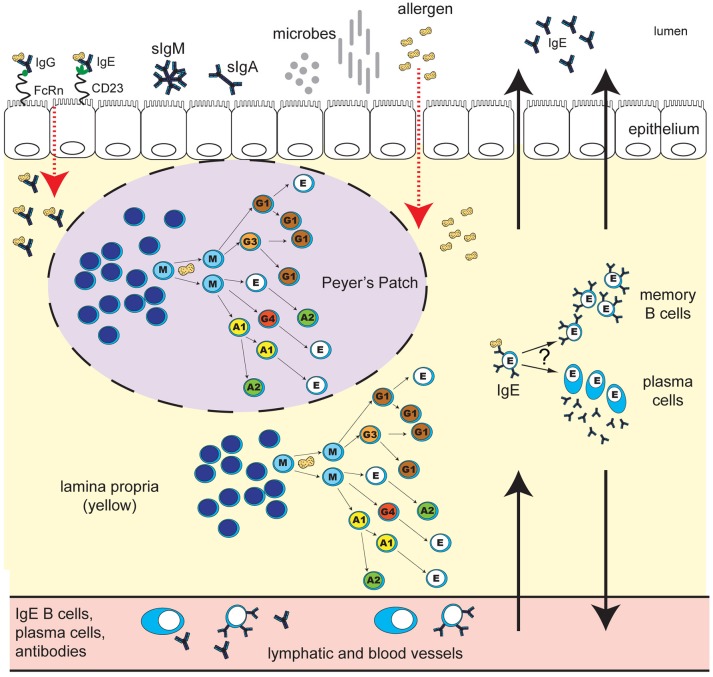
Figure 1.
Human IgE development in food allergy: unresolved questions. B cell class-switching to IgE in humans may occur from IgM, IgD, IgG3, IgG1, IgA1, or IgG4, but not from IgA2, which is downstream of IgE in the chromosomal locus. The frequency of class-switching to IgE in the gastrointestinal tract, and the microanatomical localization of these events, is unclear. Potential sites of IgE development include secondary lymphoid structures such as Peyer's Patches (purple oval with dashed line), extrafollicular sites in the lamina propria (yellow), or alternatively, other secondary lymphoid or mucosal sites in the body. What T cell help, if any, is required for IgE switching in different gastrointestinal sites is unclear. B cell exposure to food allergen proteins can occur via introduction of allergen proteins to the GALT, and possibly other tissue sites. The key routes of allergen access from the gut lumen may include epithelial barrier disruption, or transcytosis of immunoglobulin-allergen complexes via Fc receptors expressed on epithelial cells (pictured: allergen-IgE bound to “low-affinity” IgE receptor CD23, and allergen-IgG bound to FcRn). Uptake can also occur via dendritic cells and macrophages (not shown). Key questions for ongoing research include: Do B cells that class-switch to IgE in the gastrointestinal tract differentiate into memory B cells or plasma cells in situ? To what extent do daughter cells persist locally in the tissue as opposed to distributing to other sites in the body via the lymph and blood? What are the effector functions of locally-generated vs. systemic food-allergen-specific IgE? How do the microbiota influence these processes? How does the development of IgE in the gastrointestinal tract differ in individuals with food allergy compared to healthy individuals, and how are these pathways altered during immunotherapy for food allergy?