Abstract
The Cretaceous/Palaeogene (K–Pg) episode is an iconic mass extinction, in which the diversity of numerous clades abruptly declined. However, the responses of individual clades to mass extinctions may be more idiosyncratic than previously understood. Here, we examine the diversification dynamics of the three major mammalian clades in North America across the K–Pg. Our results show that these clades responded in dramatically contrasting ways to the K–Pg event. Metatherians underwent a sudden rise in extinction rates shortly after the K–Pg, whereas declining origination rates first halted diversification and later drove the loss of diversity in multituberculates. Eutherians experienced high taxonomic turnover near the boundary, with peaks in both origination and extinction rates. These findings indicate that the effects of geological episodes on diversity are context dependent and that mass extinctions can affect the diversification of clades by independently altering the extinction regime, the origination regime or both.
Keywords: macroevolution, speciation, diversity, Mammalia
1. Background
It is widely accepted that near-synchronous, often rapid, global extinctions have occurred at least five times in the Earth's history [1]. The effects of these extinction events on many organisms seemed relatively straightforward. For example, not a single non-avian dinosaur has been discovered in rocks younger than the end Cretaceous [2], approximately 66 Ma, indicating a major upheaval in the world's biota at the Cretaceous/Palaeogene (K–Pg) boundary. For many other organisms, the event may have provided ecological and evolutionary opportunities previously beyond their reach.
Fossil and molecular evidence suggest that some mammals and birds underwent radiations shortly after the K–Pg [3,4]. Studies on the diversification of mammals show that both origination and extinction rates peaked near the boundary [5], but while some mammalian clades were already accumulating diversity before the K–Pg others were declining [6,7]. These studies hint at a complex interplay between origination and extinction shaping diversity patterns of individual mammal clades during the mass extinction.
The K–Pg event is typically characterized by a rise in extinction rate [8]. Considering the scale and abruptness of this mass extinction, it should be expected that mammalian clades would have responded in similar ways, with extinction rates peaking during the K–Pg followed by increased diversification. We test this hypothesis by examining a high-resolution fossil dataset that includes the three major mammalian clades present in North America near the K–Pg boundary: Eutheria, Metatheria and Multituberculata. We estimate the rates of origination and extinction for each clade and detect contrasting, archetypical diversification patterns for each group.
2. Material and methods
(a). Dataset
We used a dataset of 188 latest Cretaceous and Palaeocene fossil assemblages (spanning from 69.9 to 55 Ma) located in the Western Interior of North America. This dataset includes information on nearly 290 genera compiled from the literature, dissertations and the Paleobiology Database (http://paleobiodb.org) [9]. The Western Interior of North America bears the richest and most extensively studied assemblages near the K–Pg; fossil occurrences are relatively well resolved, minimizing taxonomic uncertainty. Each occurrence was vetted for taxonomic consistency and taxonomic decisions were based on the most recent, most comprehensive taxonomic studies [9,10]. All occurrences were binned into North American Land Mammal ‘Ages’ (NALMAs). The dataset is available as electronic supplementary material, table S1.
To ensure that the uncovered diversification patterns were not simply the result of spatial and temporal boundaries characterizing our dataset, all analyses were repeated using a dataset of all North American mammalian occurrences retrieved from the Paleobiology Database. These analyses provide insights into the robustness of the inferred patterns to two potential sources of bias. First, this dataset includes other assemblages (outside the Western Interior of North America) and variable temporal resolution. Second, no arbitrary temporal boundaries were imposed on these data eliminating potential edge effects.
(b). Estimating origination and extinction rates from fossil occurrences
We used the PyRate framework [11] to infer origination and extinction rates and their variation through time. PyRate consists of hierarchical Bayesian models that consider fossil occurrence patterns as the result of two processes: preservation and diversification [12]. We used the recently implemented Reversible Jump Markov Chain Monte Carlo (RJMCMC) algorithm to infer the number of significant rate shifts and their temporal placement [13]. We built rates-through-time (RTT) plots from posteriors of 100 replicates of each dataset [11].
Given the heterogeneous quality of the fossil record through time, we considered time-varying preservation rates. Additionally, because the data are temporally restricted, the times of origin and times of extinction estimated using PyRate could be artificially truncated at the lower and upper limits of the time intervals. To reduce a potential edge effect on origination and extinction rates, we identified, with help from the literature and online databases, those taxa with occurrences before and after the temporal interval specifying them in the analysis. We also used the preservation rate estimated from the first and last time windows to estimate the length of potential edge bias and discarded estimates in this interval (for further details on PyRate and comparisons with other methods, see the electronic supplementary methods).
3. Results
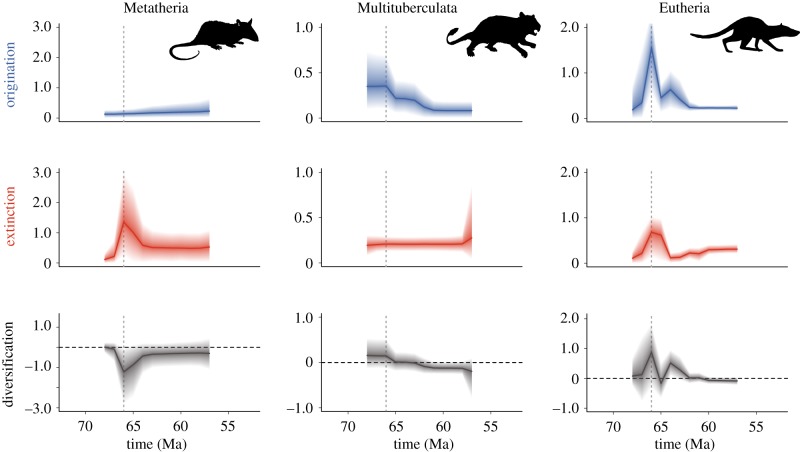
The per lineage origination rate of Metatheria remained roughly constant throughout the studied interval; however, a clear peak in extinction is identified during the K–Pg, generating a pulse of negative net diversification. The extinction rate gradually diminishes but the negative net diversification persists for more than 2 Myr (figure 1).
Figure 1.
Origination, extinction and diversification rates (Myr−1) for the three mammalian clades in North America. Solid lines and shaded areas depict mean posterior rates and 95% credible interval. Dotted lines denote the K–Pg boundary (66 Ma).
Multituberculates were diversifying prior to the K–Pg boundary, showing high origination and relatively low extinction rates (figure 1). While the extinction rate remained low, a first drop in origination around the K–Pg boundary brings the diversification of multituberculates near zero and a subsequent drop, after the K–Pg, leads to negative diversification (figure 1).
Eutherians show high origination and high extinction near the K–Pg (figure 1), resulting in high diversity turnover. Originations were higher than extinctions, except between 66 and 64 Ma. A second pulse of origination and a drop in extinction rate impinges a short burst in diversification. Around 62 Ma, origination had decreased and diversification was around zero, suggesting diversity equilibrium.
When using all North American occurrences for each group across their entire temporal range we detect patterns similar to those obtained in the main analysis for Metatheria and Eutheria, but a shallower drop in origination and a steeper increase in extinction for Multituberculata (see electronic supplementary material, figure S1). The discrepancy for Multituberculata may be explained by the lower resolution of multituberculates within the full dataset (electronic supplementary material, figure S2), which extends occurrences to stage boundaries, creating artefactual synchrony in extinction or origination times. For multituberculates and eutherians, rate estimates using alternative metrics generally converged to the mean rates obtained using PyRate, even though PyRate is more conservative in detecting shifts. These metrics were unable to obtain reasonable rate estimates for metatherians and close to the interval edges, as a result of their sensitivity to the number of occurrences (see electronic supplementary material, methods and figures S3–S5).
4. Discussion
The K–Pg episode has long been considered an unequivocal mass extinction given the massive losses in the taxonomic richness that swept entire clades [8]. However, the mechanisms whereby mass extinctions affect diversity are not homogeneous [14]. The episodes near the Devonian and Triassic boundaries, for example, were driven by a lack of origination rather than elevated extinction [8]. Similarly, during the K–Pg event, mammals underwent changes in both origination and extinction [5]. Here, we further suggest that the three major mammalian clades present in North America during this time responded quite differently to the K–Pg event.
We found three archetypical diversification patterns among the mammalian groups. Metatheria conforms to a classic mass extinction response: several extinctions temporally clustered leading to a severe drop in diversification [15]. Multituberculates underwent a diversity depletion, wherein the decrease in diversification and subsequent diversity loss was driven by declining origination rates, as seen for marine organisms during the Devonian and Triassic [8]. Eutherians show more complex rise and fall dynamics more accurately ascribed to a mass turnover event [16], with origination and extinction peaking near the same time.
Competitive displacement by eutherians has been proposed to explain the demise of metatherians [17]; however, the harsh abiotic and biotic changes near the K–Pg boundary make it difficult to infer displacement versus passive replacement. The K–Pg extinction was ecologically selective, with specialized faunivorous metatherians and insectivorous eutherians among the extinct taxa [18], whereas more generalized eutherians and multituberculates survived [19]. Tooth morphology suggests many metatherians were at the carnivore end of the diet spectrum [19] and dietary specialization could be responsible for high extinction rates.
Although our results indicate that eutherians did suffer substantial losses at the K–Pg boundary, these losses were balanced by increased origination. Diversification of resident survivors and immigrations of foreign lineages, such as plesiadapiform primates and archaic ungulates [18], followed by in situ diversification resulted in rapid accumulation of taxonomic and ecological diversity [7,20]. Lineages with plant-based diets led the diversity recovery, presumably benefiting from the expansion of angiosperm forests [18, 19]. The ecological opportunities created as incumbents were removed may also have favoured diversification and the influx of foreign lineages [3,21,22]. Towards the end of the studied interval, eutherian origination dropped and was balanced by extinction. As remarked by Alroy [5,23], the origination rate of North American mammals seems to be negatively correlated with diversity, suggesting diversity dependent dynamics [24,25].
The dietary plasticity of multituberculates [19,26] may have allowed taxa to persist, explaining low extinction rates. The ecological and taxonomic diversity of multituberculates was increasing during the latest Cretaceous [26]. However, our analyses show originations of multituberculates declined during and after the K–Pg, suggesting that unlike eutherians, losses were replaced at smaller rates over time. Although mass extinction episodes are intuitively associated with widespread extinction, origination could also be impaired by the demographic impacts of the K–Pg environmental disruption. If the populations of persisting species are driven to small sizes, the emergence and establishment of novel species become less likely [27]. As more ecological information about the taxa and the ecological context of the K–Pg is uncovered, we may be able to comprehend the mechanisms underlying differences in macroevolutionary responses among groups.
Bambach et al. showed mass extinctions can also result from low origination, being better termed ‘mass depletions’ [8]. Our results indicate that when clades are assessed individually, mass extinction events may be seen as shifts in extinction, origination or both regimes. Studies on macroevolutionary phenomena centred on broad taxonomic groups may miss a much richer macroevolutionary history that developed at finer scales.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the contributors to and organizers of the Paleobiology Database.
Data accessibility
Data are available as electronic supplementary material.
Authors' contributions
B.D.R., T.B.Q. and M.M.P. conceived the study; B.D.R. curated the data; M.M.P. and D.S. conducted analyses; all authors helped interpreting the results, writing and reviewing the manuscript; all authors approved the final version of the manuscript and agree to be held accountable for the content herein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by São Paulo Research Foundation (FAPESP nos 15/15251-4 and 13/22016-6 to M.M.P. and T.B.Q.; no. 12/04072-3 to T.B.Q.), the Doris O. and Samuel P. Welles Research Fund, UCMP (to B.D.R.) and the Swedish Research Council (2015-04748) and Knut and Alice Wallenberg Foundation to D.S.
References
- 1.Raup DM, Sepkoski JJ. 1982. Mass extinctions in the marine fossil record. Science 215, 1501–1503. ( 10.1126/science.215.4539.1501) [DOI] [PubMed] [Google Scholar]
- 2.Archibald JD. 2011. Extinction and radiation: how the fall of dinosaurs led to the rise of mammals. Baltimore, MD: Johns Hopkins. [Google Scholar]
- 3.Meredith RW, et al. 2011. Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334, 521–524. ( 10.1126/science.1211028) [DOI] [PubMed] [Google Scholar]
- 4.Ksepka DT, Stidham TA, Williamson TE. 2017. Early Paleocene landbird supports rapid phylogenetic and morphological diversification of crown birds after the K–Pg mass extinction. Proc. Natl Acad. Sci. USA 114, 8047–8052. ( 10.1073/pnas.1700188114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alroy J. 1999. The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Syst. Biol. 48, 107–118. ( 10.1080/106351599260472) [DOI] [PubMed] [Google Scholar]
- 6.Wilson GP. 2013. Mammals across the K/Pg boundary in northeastern Montana, U.S.A.: dental morphology and body-size patterns reveal extinction selectivity and immigrant-fueled ecospace filling. Paleobiology 39, 429–469. ( 10.1666/12041) [DOI] [Google Scholar]
- 7.Halliday TJD, Goswami A. 2016. Eutherian morphological disparity across the end-Cretaceous mass extinction. Biol. J. Linn. Soc. 118, 152–168. ( 10.1111/bij.12731) [DOI] [Google Scholar]
- 8.Bambach RK, Knoll AH, Wang SC. 2004. Origination, extinction, and mass depletions of marine diversity. Paleobiology 30, 522–542. ( 10.1666/0094-8373(2004)030%3C0522:OEAMDO%3E2.0.CO;2) [DOI] [Google Scholar]
- 9.Rankin BD. 2015. Paleobiogeography of latest Cretaceous and early Paleocene mammals from North America. PhD thesis, University of Calgary. [Google Scholar]
- 10.Rankin BD. 2018. New late Paleocene (late middle Tiffanian) mammals from the Roche Percée local fauna, south-eastern Saskatchewan, Canada. J. Syst. Palaeontol. 16, 361–393. ( 10.1080/14772019.2017.1296498) [DOI] [Google Scholar]
- 11.Silvestro D, Schnitzler J, Liow LH, Antonelli A, Salamin N. 2014. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Syst. Biol. 63, 349–367. ( 10.1093/sysbio/syu006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silvestro D, Salamin N, Schnitzler J. 2014. PyRate: a new program to estimate speciation and extinction rates from incomplete fossil data. Methods Ecol. Evol. 5, 1126.–. ( 10.1111/2041-210X.12263) [DOI] [Google Scholar]
- 13.Silvestro D, Antonelli A, Salamin N, Meyer X. 2018. Improved estimation of macroevolutionary rates from fossil data using a Bayesian framework. bioRχiv. ( 10.1101/316992) [DOI]
- 14.Bambach RK. 2006. Phanerozoic biodiversity mass extinctions. Annu. Rev. Earth Planet. Sci. 34, 127–155. ( 10.1146/annurev.earth.33.092203.122654) [DOI] [Google Scholar]
- 15.Raup DM. 1994. The role of extinction in evolution. Proc. Natl Acad. Sci. USA 91, 6758–6763. ( 10.1073/pnas.91.15.6758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrba ES. 1993. Turnover-pulses, the Red Queen, and related topics. Am. J. Sci. A 293, 418–452. ( 10.2475/ajs.293.A.418) [DOI] [Google Scholar]
- 17.Archibald JD. 1983. Structure of the K-T mammal radiation in North America: speculations on turnover rates and trophic structure. Acta Palaeontol. Pol. 28, 7–17. [Google Scholar]
- 18.Wilson GP. 2014. Mammalian extinction, survival, and recovery dynamics across the Cretaceous-Paleogene boundary in northeastern Montana, USA. Spec. Pap. Geol. Soc. Am. 503, 365–392. ( 10.1130/2014.2503(15)) [DOI] [Google Scholar]
- 19.Grossnickle DM, Newham E. 2016. Therian mammals experience an ecomorphological radiation during the Late Cretaceous and selective extinction at the K–Pg boundary. Proc. R. Soc. B 283, 20160256 ( 10.1098/rspb.2016.0256) [DOI] [Google Scholar]
- 20.Lillegraven JA, Eberle JJ. 1999. Vertebrate faunal changes through Lancian and Puercan time in southern Wyoming. J. Paleontol. 73, 691–710. ( 10.1017/S0022336000032510) [DOI] [Google Scholar]
- 21.Pires MM, Silvestro D, Quental TB. 2015. Continental faunal exchange and the asymmetrical radiation of carnivores. Proc. R. Soc. B 282, 20151952 ( 10.1098/rspb.2015.1952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longrich NR, Scriberas J, Wills MA. 2016. Severe extinction and rapid recovery of mammals across the Cretaceous–Palaeogene boundary, and the effects of rarity on patterns of extinction and recovery. J. Evol. Biol. 29, 1495–1512. ( 10.1111/jeb.12882) [DOI] [PubMed] [Google Scholar]
- 23.Alroy J. 1996. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 127, 285–311. ( 10.1016/S0031-0182(96)00100-9) [DOI] [Google Scholar]
- 24.Pires MM, Silvestro D, Quental TB. 2017. Interactions within and between clades shaped the diversification of terrestrial carnivores. Evolution 71, 1855–1864. ( 10.1111/evo.13269) [DOI] [PubMed] [Google Scholar]
- 25.Liow LH, Finarelli JA. 2014. A dynamic global equilibrium in carnivoran diversification over 20 million years. Proc. R. Soc. B 281, 20132312 ( 10.1098/rspb.2013.2312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483, 457–460. ( 10.1038/nature10880) [DOI] [PubMed] [Google Scholar]
- 27.Maurer BA. 1989. Diversity-dependent species dynamics: incorporating the effects of population-level processes on species dynamics. Paleobiology 15, 133–146. ( 10.1017/S0094837300009325) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as electronic supplementary material.