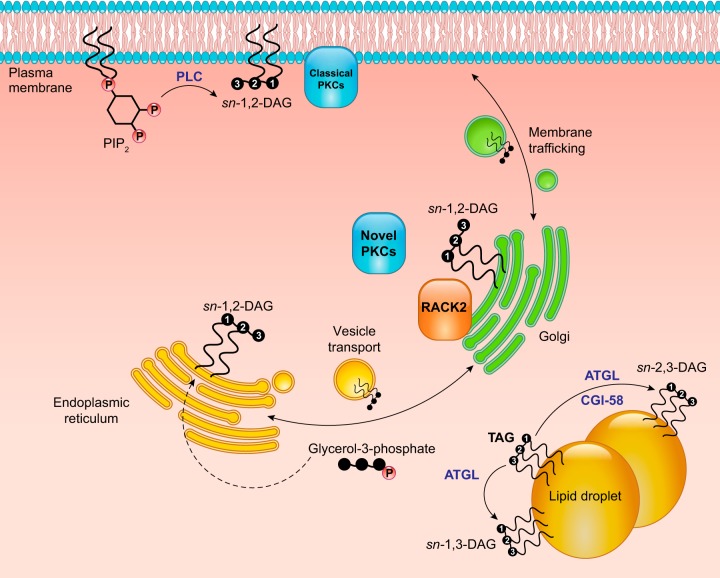
FIGURE 15.
Subcellular localization and stereoisomers of diacylglycerol (DAG). DAG is produced by the action of various enzymes at multiple intracellular sites, only a few of which are depicted. At the plasma membrane, phospholipase C (PLC) generates sn-1,2-DAG from phosphatidylinositol-4,5-bisphosphate (PIP2); this DAG is particularly critical for the activation of the classical protein kinase C (PKC) isoforms that also require the Ca2+ liberated downstream of PLC activity. The lipogenic enzymes of the Kennedy pathway are localized to the endoplasmic reticulum (ER) and produce sn-1,2-DAG. The novel PKC isoforms display significant localization to the Golgi. For PKCε in particular, this partially owes to the Golgi localization of its adapter protein RACK2. RACK2 also participates in vesicle transport between the ER and Golgi, and Golgi DAGs regulate protein trafficking to the plasma membrane. Notably, lipolytic DAG generated by the action of adipose triglyceride lipase (ATGL) at the lipid droplet is of the sn-1,3 stereoisomer and would not be predicted to activate PKC isoforms.