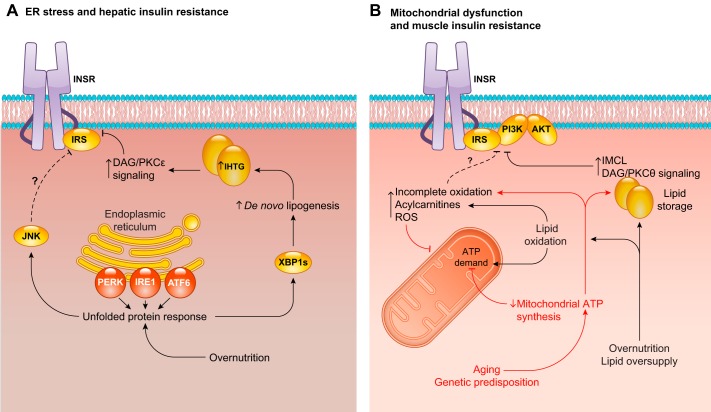
FIGURE 18.
Nutrient stress and insulin resistance. A: endoplasmic reticulum (ER) stress, manifested as the unfolded protein response (UPR), is activated in multiple models of liver insulin resistance. Accumulation of misfolded proteins in the ER lumen initiates the UPR, activating PKR-like eukaryotic initiation factor 2α kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). Key effectors of the UPR include c-Jun NH2-terminal kinase (JNK) and the mRNA splice variant of X-box binding protein 1 (XBP1s). JNK may directly impair proximal insulin signaling, although this is controversial. XBP1s transcriptionally activates the de novo lipogenic program and promotes hepatic steatosis, which may in turn drive hepatic insulin resistance through diacylglycerol (DAG)/protein kinase C (PKC)ε signaling. B: muscle insulin resistance is associated with mitochondrial dysfunction, although cause and effect relationships are not clearly defined. The red pathway describes observations made in humans: older adults and the young lean insulin-resistant offspring of parents with type 2 diabetes (T2D) display reduced mitochondrial ATP synthesis in skeletal muscle, probably reflective of reduced ATP demand as resting mitochondria operate at submaximal ATP synthetic rates. This decrease in substrate oxidation promotes lipid storage, increasing intramyocellular lipid (IMCL). IMCL accumulation may then impair proximal insulin signaling through activation of the DAG/PKCθ axis, accounting for the reduced insulin-stimulated glucose uptake observed in these individuals. Alternatively, chronic overnutrition and/or lipid oversupply increases the mitochondrial capacity for fatty acid oxidation. However, because ATP demand is relatively inflexible, this increase is not enough to match supply, leading to IMCL accumulation and increased rates of incomplete fatty acid oxidation. Incomplete fatty acid oxidation produces acylcarnitine species and reactive oxygen species (ROS). Acylcarnitines have been hypothesized to impair insulin signaling through undefined mechanisms, and ROS have broad cellular effects, including impairment of mitochondrial function. ROS-induced mitochondrial damage would then exacerbate these effects, further promoting IMCL accumulation and ROS production.