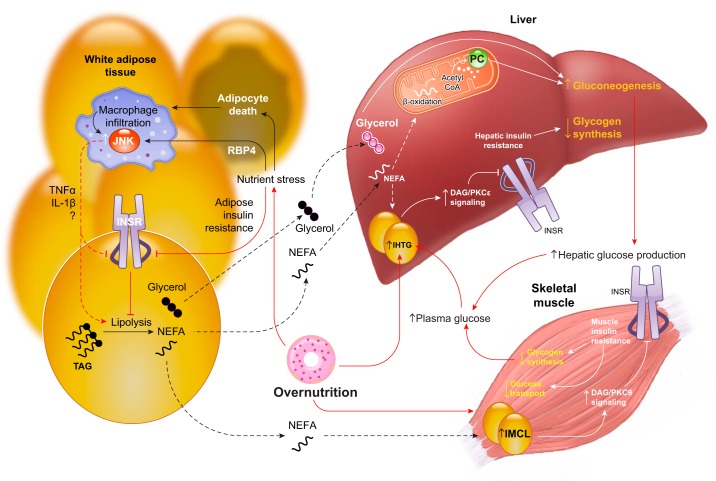
FIGURE 19.
An integrated physiological perspective on tissue insulin resistance. Chronic overnutrition is the ultimate cause of systemic insulin resistance and promotes insulin resistance by both tissue-autonomous and crosstalk-dependent mechanisms. Chronic overnutrition promotes lipid accumulation in skeletal muscle and liver, which causes insulin resistance in those tissues. Additionally, chronic overnutrition poses a nutrient stress to adipocytes, resulting in adipocyte insulin resistance and adipocyte death. Increases in the adipokine RBP4 and other proinflammatory signals lead to the recruitment of macrophages to white adipose tissue. Inflammatory signaling in macrophages, including activation of c-Jun NH2-terminal kinase (JNK), leads to the elaboration of paracrine mediators such as tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), and others. These inflammatory cytokines may increase adipocyte lipolysis either directly or indirectly by impairing insulin signaling. The increased adipocyte lipolysis of inflammation increases nonesterified fatty acid (NEFA) and glycerol turnover. This has direct (glycerol conversion to glucose) and indirect [NEFA-derived acetyl CoA activation of pyruvate carboxylase (PC)] stimulatory effects on gluconeogenesis, and also promotes accumulation of intrahepatic triglyceride (IHTG) and consequent lipid-induced hepatic insulin resistance, which impairs insulin stimulation of net hepatic glycogen synthesis. Together, these effects increase hepatic glucose production. Chronically increased lipolysis may also facilitate the accumulation of intramyocellular lipid (IMCL) and consequent lipid-induced muscle insulin resistance. The decreased glucose disposal of muscle insulin resistance increases glucose availability for the liver, which in turn promotes IHTG accumulation and worsens hepatic insulin resistance.