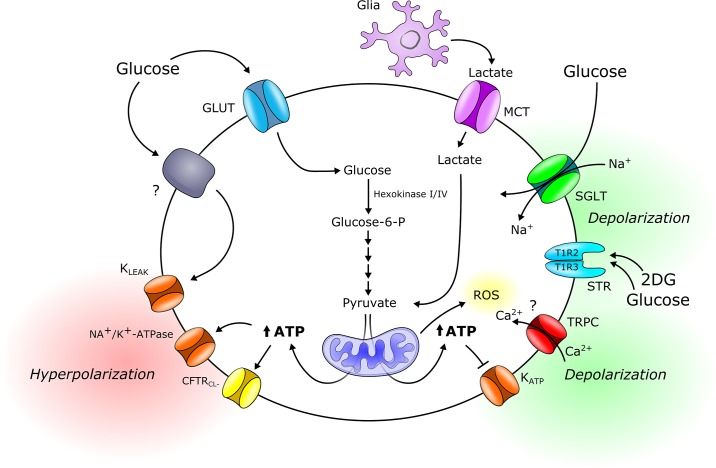
Fig. 1.
Schematic drawing of proposed neural glucose-sensing mechanisms. Glucose can be transported into the neuron by glucose transporters (GLUTs) or cotransported with Na+ by sodium-glucose transport proteins (SGLTs). The latter mechanism of entry would lead to depolarization and increased excitability of the neuron by generation of an inward current. Inside the neuron, glucose phosphorylation by glucokinase (hexokinase IV) acts as a rate-limiting step proportional to extracellular glucose concentrations. Lactate can be taken up by the neuron via monocarboxylate transporters (MCTs) to serve as a glycolytic substrate and metabolized into pyruvate. Mitochondrial uptake and metabolism of pyruvate lead to an increased ATP-to-ADP ratio and inhibition of ATP-dependent K+ (KATP) channels, resulting in plasma membrane depolarization and neurotransmitter release. Alternatively, an increased ATP-to-ADP ratio could increase Na+/K+-ATPase activity or trigger cystic fibrosis transmembrane conductance regulator (CTFRCl) channel opening, resulting in plasma membrane hyperpolarization. In orexin-expressing glucose-inhibited neurons, both glucose and 2-deoxy-d-glucose (2DG) interact with an unknown protein to trigger K+ leak (Kleak) channels, resulting in plasma membrane hyperpolarization. The metabolism-independent sweet taste receptor (STR) T1R2/T1R3 has been implicated in the glucose response of non-proopiomelanocortin (POMC) glucose-excited neurons in the arcuate nucleus (ARC). Transient receptor potential canonical (TRPC) type 3 cation channels are implicated in the glucose response of glucose-excited neurons of the mediobasal hypothalamus via a mechanism dependent on reactive oxygen species (ROS)/H2O2 production, rather than KATP channels. However, the exact mechanism in which ROS activates TRPC channels remains elusive. Glucose-6-P, glucose-6-phosphate.