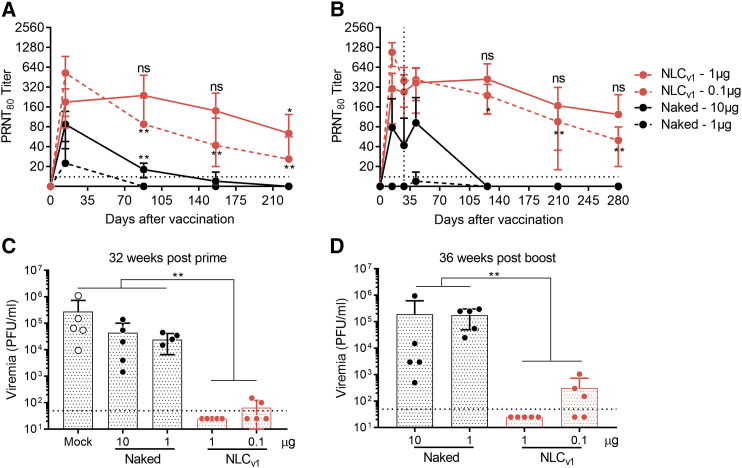
Figure 4.
Durability of Neutralizing Antibody Responses following One or Two Doses of NLC-Formulated ZIKV rvRNA
C57BL/6 mice (n = 5/group) were immunized once on day 0 (A) or on day 0 and 28 (B) via the IM route with 1 or 0.1 μg ZIKV rvRNA formulated with NLCv1, and neutralizing antibody titers at various time points were compared to mice immunized with 10 or 1 μg unformulated (naked) ZIKV rvRNA. Mice were then challenged 32 weeks post-prime (C) or 36 weeks post-boost (D) with 5 log10 PFU of ZIKV Dakar strain 41525 following antibody blockade of type I interferon as described46 and bled 4 days later to quantify viremia by plaque assay. Data is plotted as mean ± SD of each biological replicate. Data in (A) and (C) are from the same experiment, while (B) and (D) are from a separate experiment. Log10 transform of data in (A) and (B) was analyzed by one-way ANOVA with Tukey’s multiple comparison test, comparing the mean PRNT80 at each time point within each group to their respective peak titer at day 14 (in [A], NLCv1 1 μg or naked 10 μg at days 88 and 156 compared to titers at day 14, ***p < 0.0001, **p < 0.001; in [B], NLCv1 1 μg at days 126 and 209 compared to titers at day 14, *p = 0.05, ***p = 0.0001, respectively). Log10 transform of data in (C) and (D) was analyzed by one-way ANOVA with Tukey’s multiple comparison test, comparing between every group (**p < 0.005).