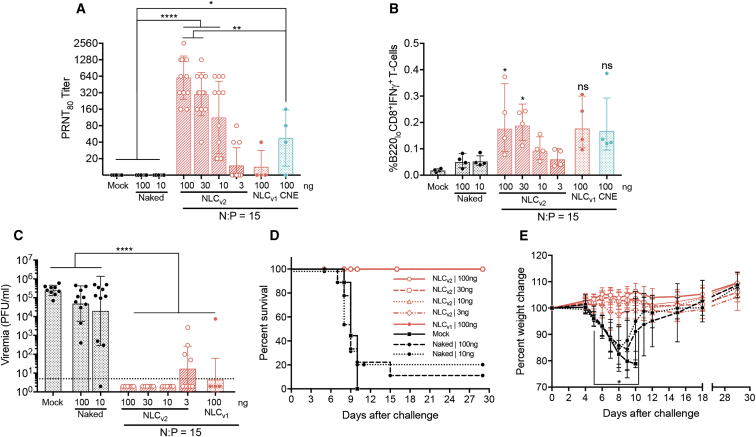
Figure 7.
Immunogenicity and Efficacy of Optimized rvRNA and NLCv2 Complexes in C57BL/6 Mice
NLCv2 was complexed with rvRNA encoding ZIKV prM and E at an N:P of 15, and 100-, 30-, 10-, and 3-ng doses were administered to C57BL/6 mice via a single IM injection (n = 14/group) and 14 days later bled to assess neutralizing antibody titers by PRNT80 (A) or euthanized (n = 4/group) to quantify percent antigen-specific B220loCD8+IFNγ+ T cells in total splenocytes (B) compared to mock-vaccinated mice, 100 or 10 ng naked rvRNA (n = 14/group), or 100 ng formulated with NLCv1 (n = 14) or CNE (n = 4) at an N:P of 15. Data are presented as individual values as well as mean ± SD. Thirty days after immunization, the remaining 10 mice per group were challenged with 5 log10 PFU of ZIKV Dakar strain 41525 following antibody blockade of type I interferon as described46 and bled 4 days later to quantify viremia by plaque assay (C). Data are presented as individual values as well as mean ± SD. Mice were monitored daily for survival (D) and weight loss (E). Data in (E) are presented as mean ± SD. Log10 transform of data in (A) and (C) was analyzed by one-way ANOVA with Tukey’s multiple comparison test (PRNT80 titers in [A] between control groups—mock and unformulated 100- and 10-ng doses and NLCv2 formulated 100-, 30-, and 10-ng doses, ****p < 0.0001, or CNE formulated 100 ng, *p = 0.04; between NLCv2-formulated 100- and 30-ng doses and CNE-formulated 100 ng, **p = 0.006; viremia titers in [C] between control groups—mock, unformulated 100- and 10-ng doses, and all NLCv2- or NLCv1-formulated doses, ****p < 0.0001). Data in (E) was analyzed by two-way ANOVA with Tukey’s multiple comparison test (on days 5–10 the percent weight change between control groups—mock or 100- and 10-ng naked rvRNA, and all formulated groups, *p < 0.05). Data in (A)–(C) are representative of two independent experiments.