Figure 5.
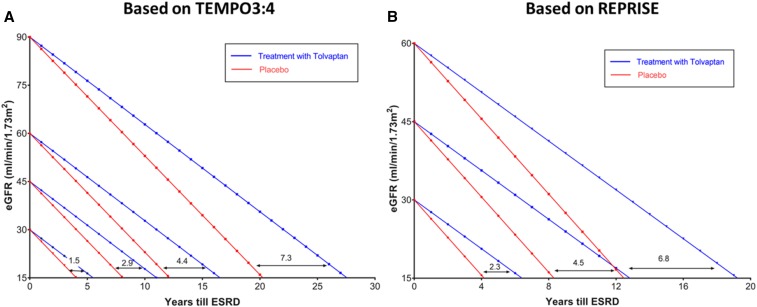
Extrapolations from the results of the TEMPO 3:4 and REPRISE trials allow estimations of the potential benefit of tolvaptan treatment in delaying the need of renal replacement therapy depending on the eGFR at the initiation of treatment. The effect of tolvaptan is predicted to be sustained and cumulative on the basis of tolvaptan trial extension and single-center experiences.4 (A) According to baseline GFR at time of treatment initiation, tolvaptan might delay reaching stage 5 CKD by 7.3, 4.4, 2.9, or 1.5 years if baseline eGFR was 90, 60, 45, or 30 ml/min, respectively. These extrapolations are made using the average decline in eGFR between placebo (−3.7 ml/min per year) and tolvaptan (−2.72 ml/min per year) groups in the TEMPO3:4 trial. (B) According to baseline GFR at time of treatment initiation, tolvaptan might delay reaching stage 5 CKD by 6.8, 4.5, or 2.3 years if baseline eGFR was 60, 45, or 30 ml/min, respectively. These extrapolations are made using the average decline in eGFR between placebo (−3.61 ml/min per year) and tolvaptan (−2.34 ml/min per year) groups in the REPRISE trial. Although this prediction model is simplistic as it assumes that all patients progress at the same slope to ESRD, it allows for visualizing of the benefit gained by patients if treated with tolvaptan early in their disease state. Predictions in (A) may underestimate the long-term benefit because the treatment effect observed in the TEMPO 3:4 trial in patients with earlier disease was less than that observed in the REPRISE trial in patients with more advanced disease. REPRISE, Replicating Evidence of Preserved Renal Function: an Investigation of Tolvaptan Safety and Efficacy in ADPKD; TEMPO 3:4, Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes.