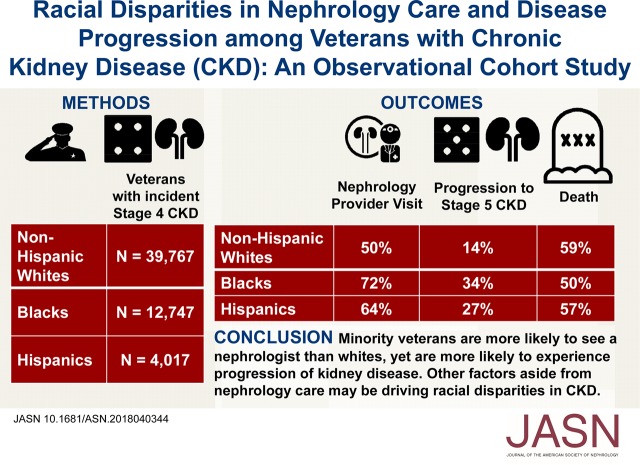
Abstract
Background
Incident rates of ESRD are much higher among black and Hispanic patients than white patients. Access to nephrology care before progression to ESRD is associated with better clinical outcomes among patients with CKD. However, it is unknown whether black or Hispanic patients with CKD experience lower pre-ESRD nephrology consultation rates compared with their white counterparts, or whether such a disparity contributes to worse outcomes among minorities.
Methods
We assembled a retrospective cohort of patients with CKD who received care through the Veterans Health Administration from 2003 to 2015, focusing on individuals with incident CKD stage 4 who had an initial eGFR≥60 ml/min per 1.73 m2 followed by two consecutive eGFRs<30 ml/min per 1.73 m2. We repeated analyses among individuals with incident CKD stage 3. Outcomes included nephrology provider referral, nephrology provider visit, progression to CKD stage 5, and mortality.
Results
We identified 56,767 veterans with CKD stage 4 and 640,704 with CKD stage 3. In both cohorts, rates of nephrology referral and visits were significantly higher among black and Hispanic veterans than among non-Hispanic white veterans. Despite this, both black and Hispanic patients experienced faster progression to CKD stage 5 compared with white patients. Black patients with CKD stage 4 experienced slightly lower mortality than white patients, whereas black patients with CKD stage 3 had a small increased risk of death.
Conclusions
Black or Hispanic veterans with CKD are more likely than white patients to see a nephrologist, yet are also more likely to suffer disease progression. Biologic and environmental factors may play a bigger role than nephrology consultation in driving racial disparities in CKD progression.
Keywords: chronic kidney disease, end stage kidney disease, nephrology, outcomes, health disparities
Visual Abstract
The strong association between black race and CKD progression has attracted great interest in identifying causes and remedies for this disparity. In comparison to white patients, black and Hispanic patients experience much higher incident rates of ESRD.1–5 As an example, Hsu et al.2 assembled a birth cohort using Third National Health and Nutrition Examination Survey data and showed that although black and white patients had a similar prevalence of CKD, black race was associated with a risk ratio of 4.8 for incident ESRD. However, among patients with CKD, it is unknown whether black patients have lower rates of nephrology consultation and, if so, whether lower consultation rates contribute to greater risk of CKD progression among black versus white patients.
Racial disparities in renal disease outcomes may be caused by diverse factors, including limited access to or use of quality health care, lower socioeconomic status, environmental toxins, health behaviors, and genetics.6–9 From a biologic standpoint, individuals who are homozygous for specific mutations of the APOL1 gene experience more rapid CKD progression, independent of diabetes status.10 These pathologic APOL1 gene mutations have been identified almost exclusively among individuals of African ancestry.11 However, race is also a social construct.12 In light of worse outcomes and lower access to high-quality care for black individuals across many nonrenal health domains, it remains important to determine whether factors such as nephrology consultation may also contribute to higher CKD progression rates among black patients.13
Nephrology care before ESRD is associated with decreased mortality after initiation of dialysis,14–16 higher rates of kidney transplantation,17 and earlier referral for vascular access.18 Kidney Disease Improving Global Outcomes guidelines recommend nephrology referral once a patient’s eGFR is <30 ml/min per 1.73 m2.19 Yet, depending on geography, 15%–50% of patients on dialysis never visit a nephrologist before initiating dialysis.14 A national examination of patients with ESRD reported that black patients had 33% lower odds of receiving nephrology care for >12 months before dialysis compared with white patients. The racial disparity in nephrology consultation before dialysis was most pronounced in large metropolitan areas. After seeing a nephrologist, black patients were also less likely to receive other key aspects of pre-ESRD care, such as receiving an erythropoietin-stimulating agent for anemia.20 However, many existing studies on health care access for advanced CKD did not examine a comprehensive cohort of patients with advanced CKD. Instead, the cohorts were conditioned on receiving dialysis because they make use of dialysis registry data (e.g., the US Renal Data System); this design may create biased inferences about quality of care and/or rely on inaccurate data.21
The Veterans Health Administration (VHA) health care system is the largest integrated health care system in the United States and provides care to a large number of racial minority patients, thereby offering an opportunity to study a diverse population of patients with CKD. Some studies have shown reduced racial disparity in health outcomes in the VHA, which does not have some of the barriers to subspecialty care that are prevalent elsewhere in the United States and that may particularly disadvantage minorities.22,23 Kovesdy et al. contrasted outcomes for a large cohort of veterans without CKD who received care in the VHA versus the general population. Black veterans had 24% lower all-cause mortality, whereas black patients in the general population experienced a 42% higher adjusted mortality versus white patients.24
The goal of this study was to evaluate if racial disparities exist in predialysis nephrology consultation among veterans with CKD using a VHA dataset with extensive data concerning laboratory tests, comorbidities, social characteristics, distance to the nearest VHA facility, and nephrology consultation. We hypothesized that black patients would be less likely to be referred for nephrology consultation and to visit a nephrologist than white patients with CKD. We hypothesized that lower rates of consultation with nephrology might contribute to worse outcomes among black versus white patients with CKD. We also hypothesized that nephrology consultation would be associated with reduced rates of progression to CKD stage 5.
Methods
The study was approved by the Institutional Review Board at the Philadelphia Veterans Affairs Medical Center.
Study Design
We conducted a retrospective cohort study using the VHA Corporate Data Warehouse and assembled cohorts of patients with incident CKD stage 3 and stage 4.25,26 We focused primarily on CKD stage 4 because the evidence base for nephrology referral is much stronger for patients with CKD stage 4.19
Study Setting and Cohort Definition
Any veteran with VHA health benefits who had an initial eGFR≥60 ml/min per 1.73 m2, data on race, and met criteria for incident CKD stage 3 or 4 between January 1, 2003 and December 31, 2014 was initially eligible for inclusion. Patients were followed until December 31, 2015 to allow for at least 1 year of follow-up. Incident CKD stage 3 was defined as having two consecutive eGFRs that were ≥30 and <60 ml/min per 1.73 m2 and were >90 days apart. The date of the second eGFR<60 ml/min per 1.73 m2 was the index date. The same approach, using an eGFR threshold of ≥15 and <30 ml/min per 1.73 m2, was used to ascertain CKD stage 4.27 eGFR values were calculated using the CKD Epidemiology Collaboration equation.28
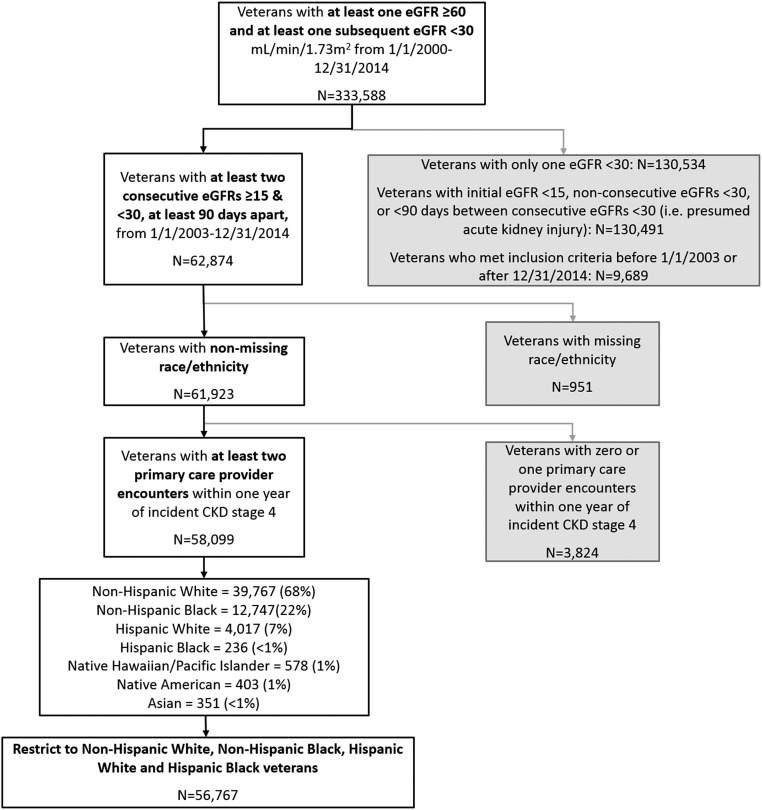
We restricted to active users of Veterans Affairs outpatient care, defined as having two or more physician outpatient primary care visits 1 year before the index date.29 Figure 1 and Supplemental Figures 1 and 2 show how the cohorts were assembled. Baseline characteristics and analysis results are available in Supplemental Tables 1–12.
Figure 1.
56,767 Veterans with incident CKD stage 4 met inclusion criteria for the study. Process for assembling cohort of individuals with CKD stage 4 who received care in the VHA. eGFR in ml/min per 1.73 m2 (calculated using the CKD Epidemiology Collaboration equation).
Exposure and Covariates
Race was the primary exposure, with a focus on comparing non-Hispanic black patients, Hispanic white patients, and Hispanic black patients to non-Hispanic white patients (reference group). Race categories are determined at enrollment in the VHA system by self-identification. In supplemental analyses, we included patients categorized as American Indian/Alaskan Native, Asian, and Native Hawaiian/Pacific Islander as well.
International Classification of Diseases, Ninth Revision codes were used to determine diagnoses of diabetes, hypertension, GN, SLE, heart failure, liver disease, chronic lung disease, malignancy, HIV, hepatitis C, hemiplegia, depression, dementia, alcohol abuse/disorder, drug abuse/drug dependence, and tobacco use before the index date (Supplemental Material).24 Systolic and diastolic BP and body mass index were calculated using all values available after January 1, 2000 and before the index date of CKD.
We derived socioeconomic status, distance to the nearest VA facility, and rurality of residency using home zip codes (Supplemental Material). VA service connection status was included as a covariate to account for differences in copays and specialty care access among veterans with and without military service-connected disabilities.30
In the subgroup of patients who had proteinuria assessed (48% of the CKD stage 4 cohort and 20% of the CKD stage 3 cohort) by 24-hour urine collection, spot urine protein, albumin-to-creatinine ratio, or urine dip at any point within 1 year before the index date, we performed exploratory analyses adjusting for proteinuria (defined as an estimated equivalent to ≥300 mg/g proteinuria).26 These analyses were considered exploratory because of the possibility that proteinuria lies in the causal pathway between race and outcomes including CKD progression. These analyses were complete case analyses. Sensitivity analyses were performed adjusting for serum phosphorus and hemoglobin, which also had substantial missing data.
Outcomes
The primary outcome was a visit to a nephrologist among patients with CKD stage 4. Secondary outcomes included nephrology provider referral, progression of renal disease to CKD stage 5, and mortality. The nephrology referral outcome was satisfied if a nephrology consult was ordered in the Veterans Affairs electronic medical record. The nephrology visit outcome was satisfied if the medical record showed nephrology appointment attendance. Progression to CKD stage 5 was defined as having two consecutive eGFR levels <15 ml/min per 1.73 m2 that were >90 days apart. In addition, we performed secondary analyses for the composite outcomes of CKD stage 5, dialysis, or renal transplantation; and doubling of serum creatinine, CKD stage 5, dialysis, or renal transplantation. We also performed sensitivity analyses restricting to individuals who had a nephrology visit, and following them from the time of the nephrology visit to CKD progression and mortality. Post hoc analyses examined for effect modification between race and age, and race and socioeconomic status.
Statistical Analyses
Descriptive statistics (median and proportion) were used to describe baseline characteristics for patients in different racial and ethnic groups. Continuous variables were compared between groups using Wilcoxon rank-sum test. Categorical and binary variables were compared using the chi-squared test.
We conducted multivariable Cox proportional hazards modeling (with censoring for death or end of follow-up) and cause-specific hazards regression (with death as a competing risk) for each of the primary and secondary outcomes (except mortality). We took into account dependence among observations within each medical center using cluster-robust estimation of the SEMs.31 We performed sensitivity analyses that included death as a composite component of each of the CKD stage 5–associated outcomes. For the outcomes of nephrology referral and nephrology provider visit, if the event occurred before or on the day of incident CKD diagnosis, these outcomes were classified as having occurred on the first day of follow-up.
Kaplan–Meier curves were generated and log-rank testing performed to assess for equality of survival distributions. For the multivariable-adjusted models, we selected variables a priori that were known to be risk factors for nephrology referral, nephrology consultation, CKD progression, and mortality on the basis of clinical judgment and previously published literature.32,33 Schoenfeld residuals were used to assess the proportional hazards assumption.
The only variable in the main analyses with substantial missing data were distance to the nearest Veterans Affairs facility, which was missing in 14% of patients. We addressed missing data with multiple imputation using chained equations to impute ten datasets.34 For the main multivariable-adjusted Cox models, the reported results represent an average of the imputed results across the datasets; the SEM accounted for uncertainty across the missing data. Multiple imputation did not meaningfully affect our findings (Supplemental Tables 3, 4, 6, 7, 10, and 11 include nonimputed results).
Statistical analyses were performed using STATA version 15 (StataCorp LP, College Station, TX), with two-sided hypothesis testing and P value of <0.05 as the criteria for statistical significance.
Results
The cohorts comprised 640,704 veterans with incident CKD stage 3 and 56,767 with incident CKD stage 4. Median follow-up was 5.7 years, and patients represented all 50 states, Puerto Rico, and Guam. Supplemental Table 1, A and B and Table 1 show baseline characteristics of each cohort. Non-Hispanic black patients with CKD stage 4 were younger (median age, 68 years; interquartile range [IQR], 61–78) and less likely to have coronary artery disease compared with non-Hispanic white, Hispanic white, and Hispanic black patients. Compared with non-Hispanic white patients, black patients, and Hispanic white patients were more likely to have proteinuria, diabetes, hypertension, liver disease, alcohol abuse, drug abuse/drug dependence, and hepatitis C.
Table 1.
Baseline characteristics by race among patients with CKD stage 4
| Characteristic | Overall Cohort, n=56,767a | Non-Hispanic White, n=39,767 | Non-Hispanic Black, n=12,747 | Hispanic White, n=4017 | Hispanic Black, n=236 |
|---|---|---|---|---|---|
| Median age, yr (IQR) | 73 (65–82) | 75 (66–82) | 68 (61–78) | 74 (65–82) | 75 (64–82) |
| Men, n (%) | 56,194 (97) | 38,618 (97) | 12,384 (97) | 3961 (99) | 231 (98) |
| Median duration of follow-up, d (IQR) | 1009 (442–1914) | 981 (426–1862) | 1113 (507–2052) | 1012 (402–1932) | 970 (252–1930) |
| Median eGFR at incident stage 4 CKD, ml/min per 1.73m2 (IQR) | 25 (22–28) | 25 (22–28) | 25 (21–28) | 25 (22–28) | 24 (20–27) |
| Medical comorbidities, n (%) | |||||
| Diabetes | 40,300 (71) | 27,584 (69) | 9356 (73) | 3190 (79) | 170 (72) |
| Hypertension | 55,153 (97) | 38,453 (97) | 12,539 (98) | 3929 (98) | 232 (98) |
| Congestive heart failure | 30,999 (55) | 22,241 (56) | 6587 (52) | 2055 (51) | 116 (49) |
| Cerebrovascular disease | 22,275 (39) | 15,941 (40) | 4538 (36) | 1699 (42) | 97 (41) |
| Coronary artery disease | 36,064 (74) | 26,778 (67) | 6732 (53) | 2425 (60) | 119 (50) |
| SLE | 308 (1) | 152 (<1) | 133 (1) | 22 (<1) | 1 (<1) |
| Liver disease | 4558 (8) | 2908 (7) | 1087 (9) | 523 (13) | 40 (17) |
| Hepatitis C | 3322 (6) | 1445 (4) | 1568 (12) | 285 (7) | 24 (10) |
| Chronic lung disease | 30,988 (55) | 22,730 (57) | 6289 (49) | 1874 (47) | 95 (40) |
| Malignancy | 1692 (3) | 1127 (3) | 433 (3) | 119 (3) | 13 (6) |
| HIV | 489 (1) | 133 (<1) | 317 (2) | 35 (<1) | 4 (2) |
| Hemiplegia | 2551 (4) | 1481 (4) | 784 (6) | 265 (7) | 21 (9) |
| Depression | 21,793 (38) | 15,125 (38) | 4710 (37) | 1859 (46) | 99 (42) |
| Dementia | 2912 (5) | 1803 (5) | 661 (5) | 422 (11) | 26 (11) |
| Social and behavioral characteristics, n (%) | |||||
| Alcohol abuse/disorder | 8073 (14) | 4514 (11) | 2860 (22) | 646 (16) | 53 (22) |
| Drug abuse/drug dependence | 3626 (6) | 1516 (4) | 1827 (14) | 260 (6) | 23 (10) |
| Tobacco use | 20,317 (36) | 14,024 (35) | 5047 (40) | 1159 (29) | 87 (37) |
| Married | 29,590 (52) | 21,684 (55) | 5491 (43) | 2290 (57) | 125 (53) |
| Median systolic BP, mm Hg (IQR) | 138 (129–146) | 136 (128–144) | 141 (133–150) | 139 (130–147) | 142 (135–151) |
| Median diastolic BP, mm Hg (IQR) | 72 (67–79) | 71 (66–77) | 78 (71–84) | 73 (68–79) | 75 (70–80) |
| Median BMI, kg/m2 (IQR) | 30 (26–34) | 30 (26–34) | 29 (25–34) | 29 (26–32) | 28 (25–32) |
| Percent service connectionb, n (%) | |||||
| <10% | 41,431 (73) | 29,471 (74) | 8837 (69) | 2953 (74) | 170 (72) |
| 10%–49% | 8759 (15) | 5777 (15) | 2373 (19) | 574 (14) | 35 (15) |
| 50%–99% | 4476 (8) | 3129 (8) | 1046 (8) | 290 (7) | 11 (5) |
| 100% | 2101 (4) | 1390 (4) | 491 (4) | 200 (5) | 20 (9) |
| Median distance to VA center, miles (IQR) | 21 (9–49) | 25 (11–53) | 14 (7–41) | 14 (7–37) | 10 (5–25) |
| Live in urban setting, n (%) | 43,311 (75) | 28,177 (71) | 11,135 (87) | 3771 (94) | 228 (97) |
| Lowest socioeconomic quartile, n (%) | 15,881 (29) | 7621 (20) | 5609 (45) | 2478 (64) | 173 (77) |
All P values were <0.001 for comparisons across the racial groups. IQR, interquartile range; BMI, body mass index; VA, Veterans Affairs.
Note that this table is limited to veterans with stage 4 CKD who are either white, black, Hispanic white, or Hispanic black.
Percent service connection denotes the amount to which disability and/or medical illness burden are associated with active military service time, and indicates additional compensation and medical services available from the VA.
Over 70% of patients had no service-connected disabilities. Non-Hispanic white patients had the lowest frequency of service-connected disabilities. Non-Hispanic black patients (median distance, 14 miles; IQR, 7–41), Hispanic white patients (median, 14 miles; IQR, 7–37), and Hispanic black patients (median, 10 miles; IQR, 5–25) tended to live closer to their respective Veteran Affairs Medical Center than non-Hispanic white patients (median, 25 miles; IQR, 11–53) for the CKD stage 4 cohort; the CKD stage 3 cohort had similar results.
Supplemental Table 2 shows outcome event rates.
Nephrology Referral and Consultation
Contrary to our hypotheses, non-Hispanic black, Hispanic white, and Hispanic black patients with CKD stage 4 were more likely to be referred to nephrology (Table 2; non-Hispanic black: adjusted hazard ratio [aHR], 1.33; 95% confidence interval (95% CI), 1.30 to 1.36; Hispanic white: aHR, 1.18; 95% CI, 1.15 to 1.22; Hispanic black: aHR, 1.26; 95% CI, 1.13 to 1.41) and to visit a nephrology provider (non-Hispanic black: aHR, 1.46; 95% CI, 1.43 to 1.49; Hispanic white: aHR, 1.39; 95% CI, 1.34 to 1.44; Hispanic black: aHR, 1.69; 95% CI, 1.52 to 1.87) compared with non-Hispanic white patients. In addition, non-Hispanic black, and Hispanic white, were more likely to have seen a nephrologist before their CKD stage 4 index date compared non-Hispanic white patients (51.2% and 40.5% versus 31.1% for white patients).
Table 2.
Cox proportional hazards models for time to nephrology referral and visit
| Nephrology Referral | |||
|---|---|---|---|
| Unadjusted CKD Stage 3a | Adjusted CKD Stage 3b | Adjusted, Death as Competing Risk, CKD Stage 3c | |
| Non-Hispanic white | — | — | — |
| Non-Hispanic black | 2.97 (2.93 to 3.02) | 2.01 (1.97 to 2.04) | 1.96 (1.93 to 2.00) |
| Hispanic white | 1.54 (1.49 to 1.58) | 1.36 (1.33 to 1.40) | 1.37 (1.33 to 1.41) |
| Hispanic black | 2.24 (2.03 to 2.48) | 1.96 (1.77 to 2.17) | 1.97 (1.77 to 2.20) |
| Unadjusted CKD Stage 4a | Adjusted, CKD Stage 4b | Adjusted, Death as Competing Risk, CKD Stage 4c | |
|---|---|---|---|
| Non-Hispanic white | — | — | — |
| Non-Hispanic black | 1.55 (1.51 to 1.59) | 1.33 (1.30 to 1.36) | 1.30 (1.28 to 1.33) |
| Hispanic white | 1.24 (1.19 to 1.29) | 1.18 (1.15 to 1.22) | 1.15 (1.12 to 1.19) |
| Hispanic black | 1.35 (1.15 to 1.58) | 1.26 (1.13 to 1.41) | 1.23 (1.09 to 1.39) |
| Nephrology Visit | |||
|---|---|---|---|
| Unadjusted CKD Stage 3a | Adjusted CKD Stage 3b | Adjusted, Death as Competing Risk, CKD Stage 3c | |
| Non-Hispanic white | — | — | — |
| Non-Hispanic black | 3.13 (3.09 to 3.18) | 2.13 (2.09 to 2.16) | 2.09 (2.06 to 2.13) |
| Hispanic white | 1.75 (1.70 to 1.80) | 1.56 (1.51 to 1.60) | 1.56 (1.51 to 1.61) |
| Hispanic black | 2.86 (2.59 to 3.15) | 2.52 (2.28 to 2.78) | 2.50 (2.25 to 2.79) |
| Unadjusted CKD Stage 4a | Adjusted, CKD Stage 4b | Adjusted, Death as Competing Risk, CKD Stage 4c | |
|---|---|---|---|
| Non-Hispanic white | — | — | — |
| Non-Hispanic black | 1.68 (1.64 to 1.72) | 1.46 (1.43 to 1.49) | 1.44 (1.40 to 1.47) |
| Hispanic white | 1.44 (1.38 to 1.50) | 1.39 (1.34 to 1.44) | 1.34 (1.30 to 1.39) |
| Hispanic black | 1.77 (1.53 to 2.06) | 1.69 (1.52 to 1.87) | 1.66 (1.48 to 1.86) |
All results are reported as hazard ratio (95% confidence interval). All P values were <0.001. —, reference group.
Unadjusted; death-censored.
Adjusted for model 1: age, sex, entry eGFR, median BP, median body mass index, hypertension diabetes, coronary artery disease, cerebrovascular disease, congestive heart failure, depression, hemiplegia, dementia, liver disease, chronic lung disease, malignancy, alcohol abuse, drug abuse, hepatitis C, smoking status, marital status, service connection, socioeconomic status, distance to Veterans Affairs medical center, clustered by medical center, using multiple imputation for missing data; death-censored.
Adjusted for model 1, addressing death as a competing risk.
These results were corroborated in the CKD stage 3 cohort (Table 2) and among other minority groups (Supplemental Table 3) and secondary analyses (Supplemental Table 4).
Additionally, in the subgroup of patients with proteinuria data, non-Hispanic black, Hispanic white, and Hispanic black patients with CKD stage 4 were more likely to be referred to nephrology after multivariable adjustment, including adjustment for proteinuria (Supplemental Table 5, middle column results).
CKD Progression
In both cohorts, non-Hispanic black, Hispanic white, and Hispanic black patients were more likely to progress to CKD stage 5 (Figure 2A, Table 3; non-Hispanic black: aHR, 1.85; 95% CI, 1.77 to 1.93; Hispanic white: aHR, 1.79; 95% CI, 1.68 to 1.92; Hispanic black: aHR, 1.75; 95% CI, 1.37 to 2.22, among the CKD stage 4 cohort) or the combined outcome of doubling of creatinine, CKD stage 5, dialysis, and kidney transplant (Table 3; non-Hispanic black: aHR, 1.68; 95% CI, 1.62 to 1.75; Hispanic white: aHR, 1.70; 95% CI, 1.60 to 1.80; Hispanic black: aHR, 1.74; 95% CI, 1.40 to 2.15, among the CKD stage 4 cohort) compared with white patients. There were similar rates of progression among the CKD stage 3 cohort across each racial group (Table 3) and among other minority groups (Supplemental Tables 6 and 7). Similar results were also obtained when analyzing progression to CKD stage 5, dialysis, and kidney transplant as a combined outcome (Supplemental Table 8) and other secondary analyses (Supplemental Table 9).
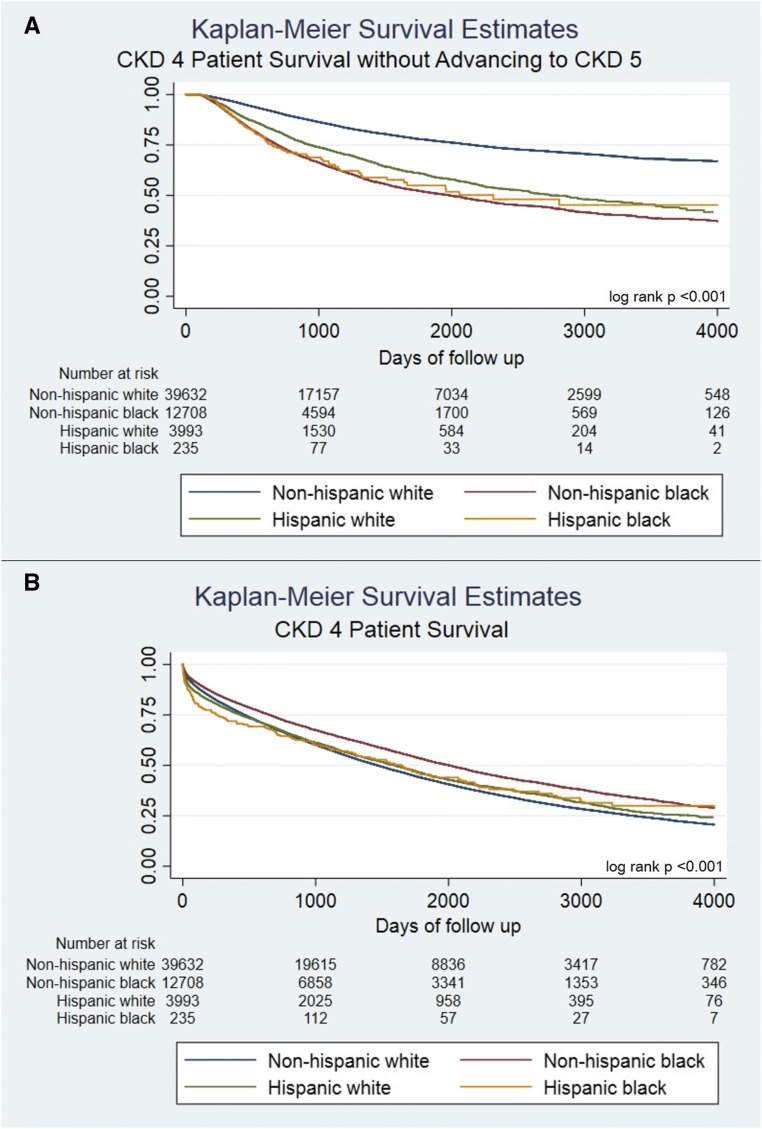
Figure 2.
Non-Hispanic white patients with CKD stage 4 experienced significantly improved survival without advancing to CKD stage 5 compared to Non-Hispanic blacks, Hispanic whites, and Hispanic blacks. Non-Hispanic blacks had the greatest overall survival. (A and B) Time to event analyses for the CKD stage 4 cohort. Risk set numbers at day 0 are smaller than totals from Table 1 because some members of the cohort achieved the outcome on day 0.
Table 3.
Cox proportional hazards models for time to CKD progression and mortality
| Progression to CKD Stage 5 | ||||
|---|---|---|---|---|
| Unadjusted CKD Stage 3a | Adjusted CKD Stage 3b | Adjusted CKD Stage 3, Only Patients Referred to Nephrologyc | Adjusted, Death as Competing Risk, CKD3d | |
| Non-Hispanic white | — | — | — | — |
| Non-Hispanic black | 5.09 (4.88 to 5.31) | 2.60 (2.48 to 2.72) | 1.64 (1.56 to 1.73) | 2.64 (2.51 to 2.78) |
| Hispanic white | 3.26 (3.04 to 3.50) | 2.50 (2.33 to 2.68) | 2.13 (1.96 to 2.31) | 2.57 (2.37 to 2.77) |
| Hispanic black | 4.22 (3.24 to 5.49) | 2.97 (2.28 to 3.87) | 2.11 (1.60 to 2.77) | 2.75 (2.02 to 3.73) |
| Unadjusted CKD Stage 4a | Adjusted CKD Stage 4b | Adjusted CKD Stage 4, Only Patients Referred to Nephrologyc | Adjusted, Death as Competing Risk, CKD Stage 4d | |
|---|---|---|---|---|
| Non-Hispanic white | — | — | — | — |
| Non-Hispanic black | 2.70 (2.60 to 2.81) | 1.85 (1.77 to 1.93) | 1.59 (1.51 to 1.68) | 1.89 (1.80 to 1.99) |
| Hispanic white | 2.07 (1.93 to 2.21) | 1.79 (1.67 to 1.92) | 1.64 (1.51 to 1.77) | 1.77 (1.64 to 1.90) |
| Hispanic black | 2.52 (1.99 to 3.21) | 1.75 (1.30 to 2.35) | 1.37 (0.96 to 1.96)e | 1.74 (1.32 to 2.30) |
| Progression to Doubling of Creatinine, CKD Stage 5, Dialysis, or Renal Transplantation Composite Outcome | ||||
|---|---|---|---|---|
| Unadjusted CKD Stage 3a | Adjusted CKD Stage 3b | Adjusted CKD Stage 3, Only Patients Referred to Nephrologyc | Adjusted, Death as Competing Risk, CKD Stage 3d | |
| Non-Hispanic white | — | — | — | — |
| Non-Hispanic black | 2.43 (2.38 to 2.48) | 1.72 (1.68 to 1.76) | 1.48 (1.38 to 1.57) | 1.74 (1.69 to 1.78) |
| Hispanic white | 1.99 (1.92 to 2.06) | 1.74 (1.68 to 1.81) | 1.87 (1.70 to 2.06) | 1.79 (1.72 to 1.86) |
| Hispanic black | 2.59 (2.27 to 2.95) | 2.06 (1.81 to 2.35) | 1.83 (1.38 to 2.42) | 2.14 (1.87 to 2.46) |
| Unadjusted CKD Stage 4a | Adjusted CKD Stage 4b | Adjusted CKD Stage 4, Only Patients Referred to Nephrologyc | Adjusted, Death as Competing Risk, CKD Stage 4d | |
|---|---|---|---|---|
| Non-Hispanic white | — | — | — | — |
| Non-Hispanic black | 2.31 (2.23 to 2.40) | 1.68 (1.62 to 1.75) | 1.37 (1.30 to 1.44) | 1.84 (1.76 to 1.93) |
| Hispanic white | 1.91 (1.81 to 2.03) | 1.70 (1.60 to 1.81) | 1.44 (1.34 to 1.56) | 1.78 (1.66 to 1.90) |
| Hispanic black | 2.36 (1.91 to 2.92) | 1.74 (1.35 to 2.23) | 1.68 (1.36 to 2.09) | 1.69 (1.31 to 2.18) |
| Mortality | ||||
|---|---|---|---|---|
| Unadjusted CKD Stage 3e | Adjusted CKD Stage 3f | Adjusted CKD Stage 3, Only Patients Referred to Nephrologyg | ||
| Non-Hispanic white | — | — | — | |
| Non-Hispanic black | 1.01 (0.99 to 1.02)e | 1.03 (1.01 to 1.04) | 1.00 (0.96 to 1.03)e | |
| Hispanic white | 1.02 (0.99 to 1.04)e | 0.96 (0.94 to 0.98) | 1.15 (1.08 to 1.21) | |
| Hispanic black | 1.22 (1.11 to 1.33) | 1.05 (0.96 to 1.15)e | 1.37 (1.12 to 1.67) | |
| Unadjusted CKD Stage 4 | Adjusted CKD Stage 4f | Adjusted CKD Stage 4, Only Patients Referred to Nephrologyg | ||
|---|---|---|---|---|
| Non-Hispanic white | — | — | — | |
| Non-Hispanic black | 0.78 (0.75 to 0.80) | 0.89 (0.86 to 0.92) | 0.92 (0.89 to 0.96) | |
| Hispanic white | 0.95 (0.91 to 0.99)e | 0.97 (0.92 to 1.01)e | 0.98 (0.91 to 1.04)e | |
| Hispanic black | 0.97 (0.82 to 1.15)e | 0.99 (0.82 to 1.19)e | 1.04 (0.80 to 1.36)e |
All results are reported as hazard ratio (95% confidence interval) unless otherwise indicated. All P values were <0.001 unless otherwise indicated. —, reference group.
Unadjusted; death-censored.
Adjusted for model 1: age, sex, entry eGFR, median BP, median body mass index, hypertension, diabetes, coronary artery disease, cerebrovascular disease, congestive heart failure, depression, hemiplegia, dementia, liver disease, chronic lung disease, malignancy, alcohol abuse, drug abuse, hepatitis C, smoking status, marital status, service connection, socioeconomic status, distance to Veterans Affairs medical center, clustered by medical center, using multiple imputation for missing data; death-censored.
Adjusted for model 1, and duration of CKD, restricted to individuals who had a nephrology visit, followed from the date of the nephrology visit; death-censored.
Adjusted for model 1, addressing death as a competing risk; reported as subhazard ratio (95% confidence interval).
P value NS.
Adjusted for model 1: age, sex, entry eGFR, median BP, median body mass index, hypertension, diabetes, coronary artery disease, cerebrovascular disease, congestive heart failure, depression, hemiplegia, dementia, liver disease, chronic lung disease, malignancy, alcohol abuse, drug abuse, hepatitis C, smoking status, marital status, service connection, socioeconomic status, distance to Veterans Affairs medical center, clustered by medical center, using multiple imputation for missing data.
Adjusted for model 1, and duration of CKD, restricted to individuals who had a nephrology visit, followed from the date of the nephrology visit.
After restricting to individuals who had at least one nephrology visit before progression, racial minorities continued to have increased risk of (1) progression to CKD stage 5 and (2) the composite outcome of doubling of creatinine, CKD stage 5, dialysis, or kidney transplant; however, the risks were attenuated compared with analyses that did not adjust for nephrology referral (Table 3).
The association between race and progression of kidney disease was similar in the death-censored model and the model treating death as a competing risk (Table 3).
The results for renal disease progression were also similar after adjusting for proteinuria in the subgroup with proteinuria results, although power to detect differences was reduced in the smaller group of Hispanic black patients (Supplemental Table 9, middle column).
Mortality
Non-Hispanic black patients with CKD stage 3 had a slightly higher mortality rate (Figure 2B, Table 3; aHR, 1.03; 95% CI, 1.01 to 1.04) compared with non-Hispanic white patients, whereas Hispanic white patients had a slightly lower mortality rate (aHR, 0.96; 95% CI, 0.94 to 0.98). In the CKD stage 4 cohort, non-Hispanic black patients had a lower risk of mortality (aHR, 0.89; 95% CI, 0.86 to 0.92) compared with non-Hispanic white patients, whereas Hispanic white and Hispanic black patients had no difference in mortality risk compared with non-Hispanic white patients (Hispanic white: aHR, 0.97; 95% CI, 0.92 to 1.01; Hispanic black: aHR, 0.99; 95% CI, 0.82 to 1.19; Supplemental Table 10 shows results among other racial groups).
Lastly, patients with CKD stage 4 and with a nephrology provider visit experienced lower mortality rates (aHR, 0.68; 95% CI, 0.65 to 0.69) compared with patients who did not see a nephrologist (Supplemental Table 11).
Effect Modification
Older age modified the effect of black race on nephrology referral and consultation, and progression to CKD stage 5 (P<0.001 for all interaction terms), such that the disparity in the risk of achieving the outcomes between black and white patients was greatest among older patients. For example, Supplemental Table 12A shows that the hazard ratio of nephrology consultation between black versus white patients was 1.79 (95% CI, 1.69 to 1.89) for patients aged ≥80 years versus 1.25 (95% CI, 1.08 to 1.44) for patients aged <50 years. Effect modification by age was not evident for mortality (P=0.15). We also did not find effect modification between socioeconomic status quartiles and race (Supplemental Table 12, A and B).
Discussion
In this large cohort involving veterans with CKD, we examined the relationships between race, nephrology consultation, and the outcomes of worsening renal disease and death. In contrast to previous registry-based studies limited to patients on dialysis,14,35 black and Hispanic veterans with CKD stage 3 or 4 were more likely to both be referred to a nephrologist and attend a nephrology consultation. Nonetheless, black and Hispanic veterans were much more likely to suffer progression to advanced renal disease. Lastly, consistent with studies involving patients on dialysis and patients with advanced CKD,32,36–40 non-Hispanic black patients with CKD stage 3 had slightly higher mortality, whereas black patients with CKD stage 4 had slightly lower mortality, compared with white patients.
Our analysis focused on the key process-of-care measure of nephrology consultation.41 Prior studies that examined dialysis patient cohorts have reported that nephrology care before ESRD is associated with lower morbidity and mortality.14,15,35,42,43 Nephrology consultation may benefit these patients through more timely placement of an arteriovenous fistula or graft instead of a dialysis catheter before dialysis initiation, as well as better control of BP, anemia, or metabolic parameters such as calcium, phosphorous, and parathyroid hormone concentrations.44–46
Our study leveraged a national cohort that was well characterized in terms of comorbidities, BP, body mass index, socioeconomic status, and distance to a health care facility. We purposefully did not restrict to patients who reached ESRD.14,15,35,42 This focus on a CKD cohort is important because of differences in GFR decline between racial groups and the need to account for the competing risk of death, which is much more likely than ESRD for patients with early-stage CKD. Blacks with and without CKD often experience steeper eGFR decline versus non-Hispanic whites.1,2,5,32,40,47 For example, Derose et al. examined data from an integrated health care system and modeled eGFR decline on the basis of serial creatinine measurement. Black patients had more rapid loss of eGFR than white patients. The authors estimated that ESRD incidence rates would be correspondingly higher among black patients with CKD, but observed that black patients had lower mortality rates (hazard ratio, 0.93; 95% CI, 0.89 to 0.98) compared with white patients.40
Contrary to our hypothesis, VHA clinicians referred black patients to nephrology at a higher rate than white patients. This result suggests the possibility that clinicians recognized the risk of more rapid GFR decline in black patients. Unfortunately, even with more frequent management by nephrologists, black patients with CKD stage 4 were 62% more likely to develop ESRD after extensive adjustment for comorbidities and socioeconomic factors. These disparities were consistent in the CKD stage 3 cohort, but the association of nephrology referral with black race was even larger in magnitude. These striking findings suggest that biologic or environmental factors drive ESRD progression through mechanisms that nephrologists cannot currently treat. These mechanisms plausibly include genetic abnormalities such as APOL1, which is believed to cause inflammatory-mediated podocyte death and has no known therapies. Other potential explanations include low access to healthy foods, or environmental toxins such as secondhand smoke or pollutants.48 Notably, our study did not aim to comprehensively assess the value of nephrology consultation, but instead assessed differential consultation rates by race and selected clinical outcomes among patients with CKD. Nephrology care may provide substantial benefits besides delaying CKD progression, such as vascular access placement or kidney transplant referral, which were not studied here.
In the CKD stage 4 cohort, black race was independently associated with an 11% lower mortality risk, whereas in the CKD stage 3 cohort, survival between racial groups was very similar. Previous studies have also reported lower death rates among black patients in the later stages of CKD.32,40 One potential explanation is that if renal disease advances faster among black patients, then by the time CKD stage 4 is reached, they may have experienced a lesser cumulative burden of CKD-related vascular morbidity compared with white patients.49 In a cohort at a single VHA center, Kovesdy et al.32 observed lower crude mortality rates in late-stage CKD and higher ESRD incidence among black patients; the authors attributed the lower mortality associated with black race to differences in clinical characteristics, with black patients having lower incidence of cardiovascular disease.
A key design feature of this study was restriction to individuals receiving care within the VHA system. Our results complement those of Fischer et al.,44 who examined a cohort of patients on incident dialysis and reported that veterans who obtained all care in the VHA had a 47% lesser likelihood of late nephrology care compared with Medicare-only users. In the VHA, individuals with advanced renal disease may benefit from the integrated electronic health care records, web-based clinical practice guidelines with CKD referral information, less of a fee-for-service environment, and waived copayments for veterans with service-connected disabilities and/or reduced means.30
The study has limitations. First, generalizability may be limited by a population of mostly older male veterans. Our CKD stage 3 and stage 4 cohorts also had a higher proportion of patients with diabetes and a lower proportion of black patients than some other national CKD cohorts, such as the National Health and Nutrition Examination Survey.50 Furthermore, the population of Hispanic black patients was small, raising the possibility of a nonrepresentative sample. Results related to the Hispanic black population should therefore be interpreted cautiously. We believe that additional surveillance related to racial disparities and quality of care is warranted in CKD populations with greater sex and socioeconomic diversity; this goal might be accomplished using data from large insurance plans or integrated health systems. Second, biased ascertainment of nephrology consultation outcomes is possible if white veterans preferentially sought consultation outside the VHA compared with black veterans. However, our cohort was restricted to active VHA health system users. Third, we lacked data on confounders such as education or health literacy. Fourth, outcomes that included dialysis and renal transplant might not capture all procedures outside the VHA. However, our cohort was selected to enrich for veterans seeking care primarily within the VHA and results were highly robust across secondary analyses that relied on “upstream” events of CKD progression, such as doubling of serum creatinine. Fifth, we did not have information on the method of serum creatinine measurement as individual VHA laboratories underwent transition to use of traceable isotope dilution mass spectrometry assays at varying times during the study period. Finally, although we performed sensitivity analyses that adjusted for proteinuria, this measurement was performed in only a minority of the cohort and using various methods (including dipstick and spot urine-to-protein ratio). On the other hand, because this analysis focuses on real-world circumstances that led to clinical decisions, the proteinuria data available are presumably the same data used to decide to refer patients to nephrology.
In conclusion, non-Hispanic black and Hispanic white veterans with CKD stages 3 and 4 were more likely to receive nephrology consultation than non-Hispanic white veterans. However, both black and Hispanic patients tended to experience more rapid renal disease progression, even when accounting for nephrology consultation and higher mortality among white patients with CKD stage 4. These findings implicate biologic and environmental factors as potentially playing a bigger role than nephrology consultation in promoting racial disparities in CKD progression.
Disclosures
None.
Supplementary Material
Acknowledgments
J.S., J.B.C., S.P.S., W.Y., and P.P.R. designed the study; J.S., J.B.C., D.K., V.P., and M.S. extracted and reshaped the data; J.S. and J.B.C. carried out the analyses; J.S. and J.B.C made the figures; all authors drafted and revised the manuscript and approved the final version of the manuscript.
J.S.’s efforts were supported by National Institutes of Health (NIH) grant 5T32DK007005-44. J.B.C.’s efforts were supported by NIH grant 1K23HL133843-02. V.P.’s efforts were supported by a Ben J. Lipps Research Fellowship grant from the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018040344/-/DCSupplemental.
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, Lin F, Vittinghoff E, Shlipak MG: Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol 14: 2902–2907, 2003 [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System : 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 4.Grams ME, Chow EK, Segev DL, Coresh J: Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis 62: 245–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer MJ, Hsu JY, Lora CM, Ricardo AC, Anderson AH, Bazzano L, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : CKD Progression and Mortality among Hispanics and Non-Hispanics. J Am Soc Nephrol 27: 3488–3497, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patzer RE, McClellan WM: Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol 8: 533–541, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton JM, Moxey-Mims MM, Eggers PW, Narva AS, Star RA, Kimmel PL, et al.: Social determinants of racial disparities in CKD. J Am Soc Nephrol 27: 2576–2595, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris KC, Williams SF, Rhee CM, Nicholas SB, Kovesdy CP, Kalantar-Zadeh K, et al.: Hemodialysis disparities in African Americans: The deeply integrated concept of race in the social fabric of our society. Semin Dial 30: 213–223, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crews DC, Liu Y, Boulware LE: Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens 23: 298–305, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al.; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DJ, Pollak MR: Genetics of kidney failure and the evolving story of APOL1. J Clin Invest 121: 3367–3374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul-Emile K: The regulation of race in science. George Washington Law Rev 80: 1115–1131, 2012 [Google Scholar]
- 13.Centers for Disease Control : CDC health disparities and inequalities report — United States, 2013. MMWR 62[Suppl 3]: 1–187, 2013 [Google Scholar]
- 14.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV: Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol 20: 1078–1085, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stack AG: Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 41: 310–318, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hao H, Lovasik BP, Pastan SO, Chang HH, Chowdhury R, Patzer RE: Geographic variation and neighborhood factors are associated with low rates of pre-end-stage renal disease nephrology care. Kidney Int 88: 614–621, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Cass A, Cunningham J, Snelling P, Ayanian JZ: Late referral to a nephrologist reduces access to renal transplantation. Am J Kidney Dis 42: 1043–1049, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Ortega T, Ortega F, Diaz-Corte C, Rebollo P, Ma Baltar J, Alvarez-Grande J: The timely construction of arteriovenous fistulae: A key to reducing morbidity and mortality and to improving cost management. Nephrol Dial Transplant 20: 598–603, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 20.Yan G, Cheung AK, Ma JZ, Yu AJ, Greene T, Oliver MN, et al.: The associations between race and geographic area and quality-of-care indicators in patients approaching ESRD. Clin J Am Soc Nephrol 8: 610–618, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JP, Desai M, Chertow GM, Winkelmayer WC: Validation of reported predialysis nephrology care of older patients initiating dialysis. J Am Soc Nephrol 23: 1078–1085, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Freeman MA, Pleis JR, Bornemann KR, Croswell E, Dew MA, Chang CH, et al.: Has the Department of Veterans Affairs found a way to avoid racial disparities in the evaluation process for kidney transplantation? Transplantation 101: 1191–1199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trivedi AN, Grebla RC, Wright SM, Washington DL: Despite improved quality of care in the Veterans Affairs health system, racial disparity persists for important clinical outcomes. Health Aff (Millwood) 30: 707–715, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, et al.: Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 132: 1538–1548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al.: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 27.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serper M, Choi G, Forde KA, Kaplan DE: Care delivery and outcomes among US veterans with hepatitis B: A national cohort study. Hepatology 63: 1774–1782, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Office of Public and Intergovernmental Affairs: Chapter 2 Service-Connected Disabilities: Federal Benefits for Veterans, Dependents and Survivors Federal Benefits for Veterans, Dependents and Survivors, 2015. Available at: https://www.va.gov/opa/publications/benefits_book/benefits_chap02.asp. Accessed December 14, 2017
- 31.Lin DY, Wei LJ: The robust inference for the Cox proportional hazards model. J Am Stat Assoc 84: 1074–1078, 1989 [Google Scholar]
- 32.Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K: Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol 4: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer MJ, Stroupe KT, Kaufman JS, O’Hare AM, Browning MM, Sohn M-W, et al.: Predialysis nephrology care and dialysis-related health outcomes among older adults initiating dialysis. BMC Nephrol 17: 103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White IR, Royston P, Wood AM: Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 30: 377–399, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Ifudu O, Dawood M, Iofel Y, Valcourt JS, Friedman EA: Delayed referral of black, Hispanic, and older patients with chronic renal failure. Am J Kidney Dis 33: 728–733, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Bleyer AJ, Tell GS, Evans GW, Ettinger WH Jr., Burkart JM: Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis 28: 72–81, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Owen WF Jr., Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: Differences by race and sex. JAMA 280: 1764–1768, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Pugh JA, Tuley MR, Basu S: Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: The emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis 23: 803–807, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, et al.: Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 62: 236–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung E, Chang TI, Chertow GM, Thomas IC, Asch SM, Kurella Tamura M: Receipt of nephrology care and clinical outcomes among veterans with advanced CKD. Am J Kidney Dis 70: 705–714, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arora P, Obrador GT, Ruthazer R, Kausz AT, Meyer KB, Jenuleson CS, et al.: Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol 10: 1281–1286, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Brooks JM, Flanigan MJ, Chrischilles EA, Pendergast JF, Hunsicker LG: Physician access and early nephrology care in elderly patients with end-stage renal disease. Kidney Int 74: 1596–1602, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Fischer MJ, Stroupe KT, Kaufman JS, O’Hare AM, Browning MM, Huo Z, et al.: Predialysis nephrology care among older veterans using Department of Veterans Affairs or Medicare-covered services. Am J Manag Care 16: e57–e66, 2010 [PubMed] [Google Scholar]
- 45.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, et al.: Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 55: 711–716, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Hall YN: Racial and ethnic disparities in end stage renal disease: Access failure. Clin J Am Soc Nephrol 7: 196–198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, et al.: Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol 22: 1327–1334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutiérrez OM, Muntner P, Rizk DV, McClellan WM, Warnock DG, Newby PK, et al.: Dietary patterns and risk of death and progression to ESRD in individuals with CKD: A cohort study. Am J Kidney Dis 64: 204–213, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, et al.: Survival advantage in black versus white men with CKD: Effect of estimated GFR and case mix. Am J Kidney Dis 62: 228–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al.; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.