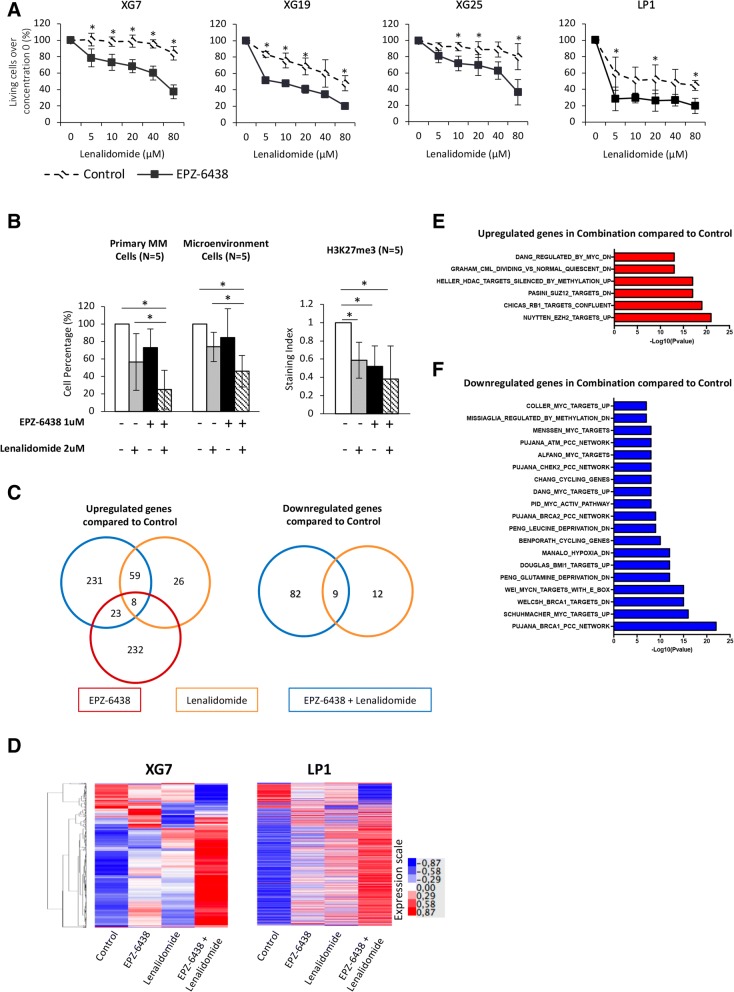
Fig. 4.
EZH2 inhibition sensitizes HMCLs and primary MMCs to lenalidomide. a HMCLs were treated 4 days with 1 μM EPZ-6438 and then cultured 4 days with graded lenalidomide concentrations. Data are mean values ± standard deviation (SD) of five experiments. * indicates a significant difference compared to control cells using t test (P ≤ 0.05). b After a 4-day pre-treatment with EPZ-6438 (1 μM), mononuclear cells from five patients with MM were treated for 4 days with 2 μM lenalidomide. The viability and total cell counts were assessed, and the percentage of CD138 viable PC and non-malignant bone marrow cells was determined by flow cytometry. Results are median values of the numbers of myeloma cells in the culture wells. Results were compared with a Wilcoxon test for pairs. c EPZ-6438, lenalidomide, and EPZ-6438/lenalidomide combination deregulated genes in MM. XG7 and LP1 HMCLs were treated with 2 μM lenalidomide (2 days) with (combination) or without (lenalidomide) prior 4 days-treatment with 1 μM EPZ-6438. Venn diagram showing overlap of genes deregulated by EPZ-6438, lenalidomide, or EPZ-6438/lenalidomide combination. d Heatmaps of RNA-seq analysis presenting expression profiles of EPZ-6438, lenalidomide, or combination target genes in XG7 and LP1 HMCLs. e, f Molecular signature of EPZ-6438 and lenalidomide combination deregulated genes compared to control in XG7 and LP1 was investigated using GSEA Database (all curated gene sets), and relevant pathways were presented (FDR q value ≤ 0.05)