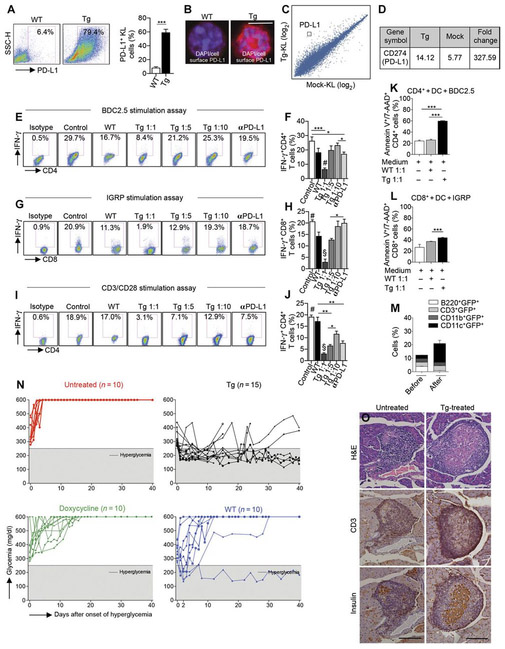
Fig. 3. Genetically engineered PD-Ll.Tg KL cells abrogate the autoimmune response in vitro and revert diabetes in hyperglycemic NOD mice in vivo.
(A) Freshly isolated murine KL cells were transduced with PD-L1 lentiviral particles, and 24 hours after transduction, cells were collected for fluorescence-activated cell sorting (FACS) analysis; representative flow cytometric analysis and quantitative bar graph of KL cells obtained from bone marrow of NOD mice before and after transduction with PD-L1 lentivirus. Experiments were run in triplicate, and statistical significance comparing WT to Tg postlentiviral PD-L1 transduction was determined by using two-tailed unpaired t test. (B) Confocal imaging of KL cells obtained from bone marrow of NOD mice pre- and postlentiviral PD-L1 transduction confirmed PD-L1 up-regulation. Histology magnification, ×63. Scale bar, 50 μm. DAPI, 4’,6- diamidino-2-phenylindole. (C and D) MA plot and PD-L1 fold change for gene expression level in KL cells obtained from bone marrow of NOD mice transduced with PD-L1 lentivirus as compared to mock-transduced KL cells, demonstrating PD-L1 up-regulation. Experiments were run in triplicate, and statistical analysis was performed using pairwise ANOVA test. (E and F) Representative flow cytometric analysis and quantitative bar graph of IFN-γ+CD4+ T cells isolated from NOD-BDC2.5 T cell receptor (TCR) Tg mice stimulated with BDC2.5 peptide in the presence of DCs (control) or upon coculture with untransduced KL cells (WT), with PD-L1.Tg KL cells (at different ratios), or with PD-L1.Tg KL cells pretreated with anti–PD-L1–blocking mAb, with the isotype control also shown. αPD-L1, anti–PD-L1–blocking mAb. (G and H) Representative flow cytometric analysis and quantitative bar graph of IFN-γ+CD8+ T cells isolated from NOD-8.3 TCR Tg mice stimulated with IGRP peptide in the presence of DCs (control), or upon coculture with WTKL cells, with PD-L1.Tg KL cells (at different ratios), or with PD-L1.Tg KL cells pretreated with PD-L1–blocking mAb. Experiments were run in triplicate, and statistical significance was determined by using two-tailed unpaired t test; #P < 0.05 versus all except Tg 1:10 and anti–PD-L1–blocking mAb (P = ns); §P < 0.05 versus all. (I and J) Representative flow cytometric analysis and quantitative bar graph of IFN-γ+CD4+ T cells isolated from normoglycemic NOD mice stimulated with soluble anti-CD3/anti-CD28 (control), or upon coculture with WT KL cells, with PD-L1.Tg KL cells (at different ratios), or with PD-L1.Tg KL cells pretreated with PD-L1–blocking mAb. PD-L1.Tg KL cells strongly abrogate the CD4- and CD8-restricted autoimmune response and anti–CD3/CD28-dependent T cell stimulation in vitro. All experiments were run at least in triplicate, and statistical significance was determined using two-tailed unpaired t test; #P < 0.05 versus all except WT (P = ns); §P < 0.05 versus all. (K and L) Naive CD4+CD25− T cells isolated from BDC2.5 TCR Tg NOD mice or CD8+ T cells from 8.3 TCR Tg NOD mice and stimulated with BDC2.5 or IGRP islet peptides and CD11c+ DCs were cocultured with KL cells (WT) or PD-L1.Tg KL cells, and the rate of apoptosis of CD4 or CD8T cells was assessed by flow cytometry. PD-L1.Tg KL cells’ effect on cell death in autoreactive CD4+ and CD8+T cells as compared to WT KL cells. Experiments were run in triplicate, and statistical significance was determined by using two-tailed unpaired t test. (M) Quantitative bar graphs for lymphoid and myeloid markers of isolated KL cells before and after lentiviral transduction. GFP+, green fluorescent protein. (N) Newly hyperglycemic NOD mice were treated with WT KL cells, with PD-L1.Tg KL cells, and with doxycycline or were left untreated. (O) Representative immunohistochemical hematoxylin and eosin (H&E) analysis and CD3/insulin staining in serial pancreatic islet tissue sections from PD-L1.Tg KL cell–treated or untreated newly hyperglycemic NOD mice. Histology magnification, ×20. Scale bars, 200 mm. *P < 0.05; **P <0.01; ***P < 0.0001.