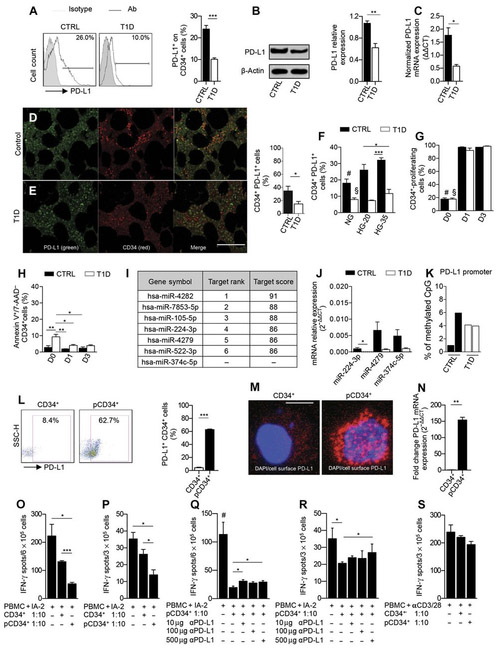
Fig. 7. The PD-L1 defect is evident in HSPCs from T1D patients.
(A) Representative flow cytometric and quantitative bar graph of PD-L1+CD34+ cells from patients with T1D as compared to healthy controls (n = 10 from each group), and statistical significance was performed by using two-tailed unpaired t test. (B) Western blot analysis and (C) qRT-PCR confirmed the PD-L1 defect in CD34+ cells from T1D patients (n = 3 in each group), and statistical significance was performed by using two-tailed unpaired t test. (D and E) Confocal imaging and quantitative bar graph of bone marrow sections obtained from T1D patients and healthy controls showing PD-L1 (green) and CD34 (red) staining; the quantification of the orange-stained bone marrow element was performed by ImageJ. Data are representative of n = 5 sections per group, and statistical significance was performed using two-tailed unpaired t test with Welch’s correction. Histology magnification, ×63. Scale bar, 40 μm. (F) Bar graph depicting the percentage of PD-L1 on CD34+ cells obtained from peripheral blood ofT1D patients or healthy controls at baseline or cultured for 3 days in normal glucose, in 20 mM, or in 35 mM high glucose (n = 3 samples from each group), and statistical significance was performed using two-tailed unpaired t test; #P < 0.05 versus all except T1D HG-35 and CTRL HG-20; §P < 0.05 versus all except T1D HG-35 (ns) and T1D HG-20 (ns). (G) CFSE-based proliferation assay of peripheral CD34+ cells obtained from T1D and healthy control patients at baseline and after 1 and 3 days of culture (n = 3 samples from each group), and statistical significance was performed using two-tailed unpaired t test; #P < 0.0001 versus all except T1D-D0; §P < 0.0001 versus all except CTRL-D0 (ns). (H) Frequency of apoptosis of CD34+ cells obtained from T1D and healthy control patients at baseline and after 1 and 3 days of culture. Experiments were run in triplicate, and statistical significance was performed using two-tailed unpaired t test. (I) Table of human miRNAs, discovered by bioinformatic approach, involved in the regulation of PD-L1 expression. (J) qRT-PCR showed differentially expressed miRNA in human CD34+ cells obtained from T1D patients as compared to controls (at least n = 5 samples from each group), and statistical significance was performed using two-tailed unpaired t test with Welch’s correction. (K) DNA methylation status of the PD-L1 gene promoter in peripheral CD34+ cells obtained from T1D patients as compared to healthy controls (n = 2 samples from each group). Statistical significance was performed using two-tailed unpaired t test with Welch’s correction. (L) Representative flow cytometric and quantitative bar graph of PD-L1 expression on peripheral CD34+ cells from T1D patients pre- and postpharmacological modulation with a cocktail of small molecules. Experiments were run in triplicate, and statistical significance was performed using two-tailed unpaired t test. (M) Confocal imaging of PD-L1 expression on CD34+ cells from T1D patients pre- and postpharmacological modulation. Histology magnification, ×63. Scale bar, 50 μm. (N) PD-L1 expression fold change in pCD34+ after 24 hours and in vehicle-treated CD34+ cells as assessed by RT-PCR. Experiments were run in duplicate, and statistical significance was performed using two-tailed unpaired t test. (O to R) Quantification of IFN-γ–producing cells where human PBMCs from T1D were challenged with IA-2 in the presence of unmodulated CD34+ or pCD34+ cells with or without an anti–PD-L1–blocking mAb. Experiments were run in triplicate, and statistical significance was performed using two-tailed unpaired t test. Experiments were performed at least in triplicate; in (R): Data related to anti–PD-L1 treatment were performed in duplicate, and statistical significance was performed using two-tailed unpaired t test. (S) Quantification of IFN-γ–producing cells where human PBMCs from T1D already stimulated with anti-CD3/anti-CD28 were cocultured in the presence of unmodulated CD34+ or pCD34+ cells with or without an anti–PD-L1–blocking mAb. Experiments were run in duplicate, and statistical significance was performed using two-tailed unpaired t test. Data are expressed as means ± SEM. *P < 0.05; **P <0.01; ***P < 0.001. #P < 0.05 versus all.