Abstract
Tissue engineering as a high technology solution for treating disc’s problem has been the focus of some researches recently; however, the upcoming successful results in this area depends on understanding the complexities of biology and engineering interface. Whereas the major responsibility of the nucleus pulposus is to provide a sustainable hydrated environment within the disc, the function of the annulus fibrosus (AF) is more mechanical, facilitating joint mobility and preventing radial bulging by confining of the central part, which makes the AF reconstruction important. Although the body of knowledge regarding the AF tissue engineering has grown rapidly, the opportunities to improve current understanding of how artificial scaffolds are able to mimic the AF concentric structure—including inter-lamellar matrix and cross-bridges—addressed unresolved research questions. The aim of this literature review was to collect and discuss, from the international scientific literature, information about tissue engineering of the AF based on scaffold fabrication and material properties, useful for developing new strategies in disc tissue engineering. The key parameter of this research was understanding if role of cross-bridges and inter-lamellar matrix has been considered on tissue engineering of the AF.
Keywords: Annulus fibrosus, Tissue engineering, Biomechanical properties, Biochemical properties, Intervertebral disc
Introduction
Low back pain (LBP) is one of the major health problems in western countries that greatly affects the quality of patients’ life and has been the subject of several clinical research studies. The relationship between LBP and disc degeneration [1–6], herniation [7, 8], nutrition [9–12] and other external factors, such as mechanical loading larger than physiological limits were well documented [13–21]. Recently, tissue engineering as a high technology solution for treating disc’s problem has been the focus of research for several studies; in fact, it has been suggested as the next step for finding a solution [18, 22].
To achieve a better insight of the tissue engineering applications for the treatment of the annulus degeneration, an accurate understanding of the local chemical and mechanical environment (sometimes called mechano-chemical mutual impacts) around the disc cells in the annulus as well as its anatomy and physiology is required.
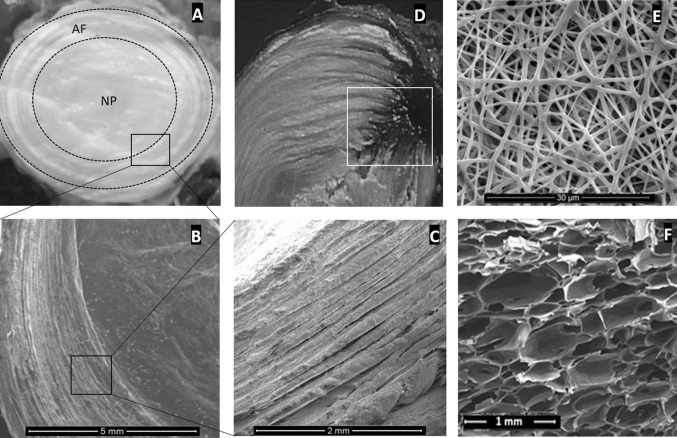
As it was presented in Fig. 1—including the AF and NP structure in different magnifications, a herniated disc, and two different tissue engineered scaffolds—at the macro scale (mm or tissue range), the AF forms the outer boundary of the disc, consisting of almost concentric collagen-matrix composite rings [1].
Fig. 1.
The AF and NP structure of an intervertebral disc in different magnifications (A, B, C), black squares show the magnified region. White square shows the NP extrusion through the AF (D), the place needs to be carefully focused for the AF treatment. Images “E” and “F” show two different scaffolds (E: nano-fibres and F: silk-base porous structure) that may be used for the torn AF re-construction
The cartilaginous extracellular matrix of the annulus contains collagen fibers whose orientation imparts material anisotropy [23]. At the micro scale, the annulus has a complex structure, with few mesenchyme cells embedded in an extracellular matrix [24] that synthesis collagen and aggregating proteoglycans [25]. The matrix composition and organization alter over time, with the cellular repair response being inadequate; as a result, the degraded matrix would no longer be able to carry the external loads effectively, leading to onset of disc degeneration [3]. Disc degeneration consists of a complex interaction of mechanical [17, 26], biological [27] and chemical [28] changes within the IVD, where the AF and NP boundary becomes less distinct as the NP losses its gel-like property and develops fibrotic changes. Disorganization of the AF and inter-lamellar matrix (ILM) including collagen and elastin disorders and irregularities of annular lamellae are other symptoms of degeneration [29].
Herniation occurs subsequent to ILM failure and affected the integrity and adhesion between annular layers [30]. The pattern of herniation clearly demonstrates that the posterior part of the AF is more vulnerable to structural failure due to weak inter-lamellar cohesion [31], low annular wall thickness [32] and a relatively large number of incomplete lamellae in adjacent layers Based on abrupt changes in the disruption pattern from intra- to inter-lamellar, the distinct lack of inter-lamellar connectivity caused concentric tears by mechanical factors rather than degeneration [33].
A fundamental understanding of ILM structure-function behavior is important for determining the complex loading conditions under which the AF is at risk for delamination and subsequent tissue disruption that may lead to degeneration. Therefore after herniation, clinical treatments should aim to strengthen and repair the AF to confine the NP, as well as target its rehydration and regrowth [34, 35]. Current treatments (invasive or conservative) for degenerated disc are not able to restore its original function and structure [36]. Tissue engineering techniques have emerged as a promising therapeutic approach, by totally or partially replacing the degenerated disc with scaffold-cells implants [36–40].
Based on the literature review it was found that scaffold surface modification [41–43], cell and cellular activities [24, 40, 44–59], cell bioengineering tips including cellular migration, cell’s biology and phenotype, cell and matrix metabolism and scaffold free constructions as a common areas between engineers and biologists [24, 44, 48, 51, 52, 59], cellular and intercellular mechanics [24, 45, 49, 54–56], preparation methods for cell culture [46, 53, 56–58] and cell – extracellular matrix interaction [40, 47, 50, 53, 56] has been focused by researches in disc’s tissue engineer activities. In some other studies scaffold structure [60–71], material [72–86], design [87–97] and its biomechanical properties [98–105] mainly considered as an important factor toward the AF tissue engineering. Some researchers presented hybrid systems whose function was not only tissue engineering but also delivery of bioactive agents for degeneration or its consequent (i.e. pain) treatment [106, 107]. Clinical application of tissue engineering in laboratory scale and their challenges was another point of view [22, 23, 27, 39, 108–115].
The requirements, achievements and challenges in the AF tissue engineering had indicated before; however, some gaps were found in this rapidly emerging field of research. The main important question that previous reviews have not answered yet is how fabrication, biomaterial selection and mechano-chemical testing of the artificial AF scaffold mimic the integrity of adjacent lamellae of the AF. Therefore the main reason of this review study was to find out whether the role of inter-lamellar matrix has had noticed on selecting biomaterials and fabrication processes or had influenced the mechno-chemical evaluation methods. The aim of this study was firstly review the most relevant journal articles published in the international scientific literature about tissue engineering of the AF based on scaffold fabrication and material properties and the second to find out what sort of activities performed to help artificial scaffold mimicking lamellae’s integrity. This review could become a comprehensive resource for researchers working in the field of the AF scaffold fabrication and evaluation.
The literature review was undertaken through an online search using the databases of Web of Science Core, PubMed (NLM) and Science Direct; articles had to be written in English language, published in peer-reviewed journals. The search terms used were as follows: “annulus fibrosus” AND/OR “intervertebral disc” AND “tissue engineering”, covering the papers published in the period from the early 1980s to 2015.
Scaffold fabrication methods
Tissue engineering scaffold fabrication of the AF has been challenging due to its complex structure. Electrospinning, bio-printing, stereo-lithography and producing porous scaffolds based on chemical processing has been introduced as some of the methods for the AF tissue engineered scaffold preparation. Nano-fibrous scaffolds usually were fabricated by electrospinning and wet spinning [36, 82] in many studies, where mandrel rotational speeds and applied voltages varied related to the materials’ specifications (see Table 1).
Table 1.
Electrospinning conditions in different studies
| Material | Mandrel speed | Applied voltage (KV) | References |
|---|---|---|---|
| Polyurethane | 1200 rpm | 20 | [51] |
| 1000 rpm | 5–10 | [77] | |
| 1250 rpm | 18 | [104] | |
| – | 15 | [125] | |
| – | 12 | [126] | |
| Polyurethane/collagen/chitosan | 4000 rpm | 18 | [127] |
| Poly(ε-caprolactone) | 10 m/s | 13 | [106] |
| 500–2000 rpm | 12 | [83] | |
| 300 rpm | 8 | [98] | |
| 30,000 rpm, 11 m/s | 23 | [50] | |
| 10 m/s | 13 | [73] | |
| 10 m/s | 13 | [99, 100] | |
| 7500 rpm | 12 | [47, 111] | |
| Poly(L-lactic acid) | – | 16 | [84, 123] |
| – | 15 | [74] | |
| Collagen/chitosan/poly(ethylene oxide) | 10 m/s | 5–30 | [124] |
Some presented scaffold fabrication methodologies like 3-dimensional bio-printing and micro-sterolithography (µSL) allowed prefabrication of anatomically relevant scaffolds in a layer-by-layer process [42, 80]. These methods were patient-based CAD modelling process and obtained dimensions from MRI or CT scans [116]. In order to successfully apply these methods, light penetration depth control and residual strains in different layers [117–119]. Using a winding machine with custom-made modifications in rotation and sliding assemblies, some researchers fabricated silk based scaffolds. In this method the angle between slide and rotation directions which manipulate the direction and maintain the angle of winding fibers, respectively, had been mentioned as two important parameters that formed the scaffold structure [60].
Contracted AF(collagen)-NP(alginate) method was used to prepare a scaffold for the whole disc replacement. This method conducted by the NP and AF dimension measurement and simple molding method. Accurate measurement and preparing too many solutions with different concentrations had to be considered [25, 87]. This method is based on generating collagen fibril structures by contracting collagen gels with different boundary conditions [87].
It was reported that porous silk scaffold that prepared by chemical processing of cocoons could be used as the AF tissue engineering. Protein extraction (Sercin), Lyophilization and special structure induction (mostly β-sheet) were the main steps in the method [62, 73, 78, 79, 107, 120–124]. The ability of silk fibers to be used as a substitution for collagen is almost new subject in tissue engineering researches [125–127]. The silk-based nano-fibrous scaffolds prepared under an all-aqueous process in ambient conditions introduced simple but highly appreciated method to the future use of these material systems in tissue studies [128–130].
Demineralized bone particle (DBP) gels had been used as an injectable scaffold to substitute intervertebral discs. DBP gel-shaped scaffold were fabricated with different percentages of DBP powder and acetic acid, including pepsin [83]. Photochemical crosslinking collagen scaffold, which cured by laser irradiation [88], riboflavin induced collagen, which was known as high density collagen gels [89], Genipin-crosslinked fibrin as an injectable adhesive [95] were introduced as intra injectable scaffold, as well.
Alginate and poly lactic acid base scaffolds (sometimes called shape-memory scaffolds), fabricated by following the sequences of crosslinking process (carbodiimide chemistry), unreacted chemical reagents removal, freeze-drying process and cell seeding [19, 74, 82, 90, 96].
The fabrication of poly lactic acid foams containing bio-glass scaffolds were made using the thermal induced phase separation (TIPS) process [75]. It was claimed that TIPS process supports fabrication of foam like scaffold with tailored porosity appropriate to the tissue concerned [131]. TIPS consisted of a sequences of polymer and bioglass-solvent mixture preparation, Lyophilization process, freezing and vacuum drying processes [132].
Freeze drying was a simple method used by some researches to convert the collagen-GAG slurries into porous scaffold [66] or at the end of the solvent casting/salt leaching technique [68, 81] or film casting [85]. Usage of this method of fabrication was limited to lab activities (i.e. proliferative and biosynthesis activity of the AF cells) due to poor mechanical properties.
Biomaterials selection
Selecting materials for fabricating scaffold is still a big concern due to the AF complex structure and physic-chemical properties. Efforts to produce AF tissue in vitro have involved various materials.
Silks which were introduced as protein polymers differ widely in composition (amino acid sequence and type), properties and structure depending on source and the most bio-oriented ones forms from the silkworm and spiders [120, 125]. Silkworm (B.mori silk) fibers are composed of core filament protein (fibroin) consist of highly organized crystalline region and the gum-like protein (sericin) that surrounded the fibers. New approaches for utilizing silk fibers in the AF scaffold leaded to recombinant silk-collagen fibers fabrication. Linking silk’s repeating sequences to a collagen domain preserved silk’s useful properties, while adding selective bioactive functions [125]. Silk fibroin-chitosan hybrid had been evaluated as a tissue engineered scaffold, where biomechanical and biochemical correlation showed some mechanical properties enhancement [126]. Based on silk biocompatibility and mechanical properties most of researches had focused on its application on scaffold construction and impact of scaffold characteristics on cell response rather than presenting new generation of silk base materials [62, 63, 73, 122, 124, 128, 129, 133].
Polyurethanes (PU) with bioactive, biocompatible and biodegradable characteristics have been considered as a substitution for the degenerated or damaged AF [97]. The possibility of managing biodegradation process has made this material a good candidate for the AF scaffold [134]. Aliphatic ester linkages [135, 136], environmental stress cracking [137], temperature and humidity [134], enzyme presence in biological environment [138] and calcification [139] were the sources of the degradation that altering PU structure. Surface modification of PU nanofibers with anionic oligomers [97, 140], coating nanofibers of polyurethane with fibronectin [141] and addition of collagen to PU nanofibers during electrospinning process [142] were valuable efforts on presenting new biomaterials for the AF tissue engineering purposes.
Poly(D, L-lactic acid), its copolymer with poly(glycolic acid) [81] and its combination with bioglass (usually in filled-composite foam form) being considered as tissue engineered scaffold [131]. Collagenous scaffold that crosslinked in situ with riboflavin at 468 nm (1400 KWcm−2) for 40 seconds were injectable and have been used to repair defects made to the AF [25, 101]. In the presence of ammonia as a crosslinking agent, argon laser at 514 nm and 0.2 W for 100 seconds was used for collagen gelation [88]. Collagen fibers usually harvested from animal tail tendon and got ready to used subsequence to some chemical and physical procedures [87, 143]. Collagen-chitosan composite blended with polyethylene oxide (PEO) was used in some researches [144]. Chitosan compatibility with glycosaminoglycan structure held superior biological properties at relatively low cost, where PEO affected fabricating process as a plasticizer [144, 145]. Collagen coated silicon membrane was used as a temporary scaffold enable researchers to develop tensile pre-strained AF cells for tissue regeneration study in vitro [103]. Poly caprolactone, was widely used as tissue engineered scaffold, alone [100] or as a blend [70]. In one research Poly (polycaprolactone triol malate) introduced as the AF’s biocompatible scaffold whose degradation capability and mechanical properties were tailor-made in accordance with pre-polymerization process [69].
Scaffold biomechanical properties
Silk is environmentally stable due to the structural crystallinity, protein hydrophobicity and the extensive hydrogen bonding; however its unique biomechanical properties have arose from the nanoscale features and conformational polymorphism originated from oriented β-sheet crystals [62, 146], stress alignment of the chains and properties of crystalline-amorphous interface [120]. With regards to two weeks cell culture on Silk fibroin-chitosan hybrid’s scaffold it was shown that scaffold’s compressive modulus was positively correlated with collagen and Glycosaminoglycan (GAG) content [126]. Mechanical study of cross linked silk fibroin fibers with chondroitin sulphate (CS) which assessed in compression mode implied on about 9-fold increase in modulus compared with non-cross-linked construct and 1.4-fold higher by the week four after cell culture for cross-linked structure [60]. Not surprisingly, crosslinking with CS increased CS-silk construct stiffness approximately twice higher than silk construct [60].
It was shown that biodegradation affected mechanical properties of polyurethane scaffold, initial modulus followed by initial wetting decreased by about 5 times, ultimate stress (MPa) decreased from 6 to 2 during four weeks wetting [97]. Surface modification of PU nanofibers with anionic oligomers (AO) affected mechanical properties of scaffold [97] as well as its physic-chemical properties [140]. Also, relation between surface modifications with matrix proteins, provided molecular and topographical cues that permit the AF cells to orient parallel to scaffold fibers [41]. Addition of AO increased scaffold’s surface polar characteristic, while reducing contact angle (≈ 40%) affected cell attachment, positively [97, 140, 147]. On the other hand coating PU nanofibers with fibronectin (a matrix protein) resulted in greater collagen synthesis and accumulation indicated that cell shape and adhesion to scaffold appeared to be correlated to matrix production [141].
Study the effect of collagen addition on PU nanofibers during electrospinning process showed that scaffold mechanical properties depended on the collagen percentages, whose increment resulted in modulus, tensile strength and breaking strain decrease [142]. Tensile strengths ranged from 2 MPa to 13 MPa and breaking strains from 160 to 280%. Electrospun PU had a tensile strength of 13±4 MPa and a breaking strain of 220±80%. Incorporation of collagen leaded to significant decrease in tensile strength as well as reduction in modulus; however, no meaningful correlation between collagen content and breaking strain were observed [148].
Some studies focused on PU nanofibers alignment’s effects on scaffold properties [41, 84, 97, 140, 148] and cell migration [149]. It was observed that most cells migrated along the fiber orientation direction on the uni-directionally aligned fibers and could travel among different fibers and change movement directions back and forth [133, 149]. Also it was proved that applying tension to PU scaffold during cell culture affect cell proliferation, alignment and morphology that suggested tensile strain was required to generate properly formed AF tissue [84]. Electrospun PU scaffolds with random and aligned nanofibers presented different mechanical properties, where aligned structures were found to have higher tensile strength and modulus (σ=14±1 compare to 1.9±0.4 (MPa) and E=46±3 compare to 2.1±0.2 (MPa)) prior to degradation [97]. Also the tensile strength of the aligned nano-fibrous scaffold showed significant differences between parallel (14±0.6 MPa) and perpendicular (5.1±1 MPa) directions; moreover comparison of strain at break between scaffold of parallel and perpendicular alignment showed an even sharper difference (60±15.5 and 8±1) [142].
Comparing PCL and PU mechanical and biochemical properties demonstrated that structural orientation had significant impact on properties improvement. It was shown that oriented PU scaffolds had higher yield strength and in contrast PCL oriented ones were stiffer [70]. Mechanical properties of the AF scaffold’s materials listed in Table 2.
Table 2.
Selected mechanical properties of the AF scaffolds’ candidate
| Material | UTS (MPa) | % Strain at break | E (GPa) | References |
|---|---|---|---|---|
| Silka | 610–690 | 4–16 | 15–17 | [132, 62] |
| Collagen | 0.9–7.4 | 24–68 | 0.0018–0.046 | [132, 145] |
| PLAb | 28–50 | 2–6 | 1.2–3 | [132] |
| PCLc | – | 7–10 | 0.055–0.06 | [77, 98] |
| PUc | – | 26–31 | 0.020–0.030 | [77] |
aFrom silkworm, individual filament following sericin extraction
b50,000 < Mw < 300,000
cnanofiber
Biochemical analysis
Investigating structural and mechanical properties’ effects on deposition and orientation of matrix [60], cell culture, proliferation and interaction with the structural elements and gene related evaluation [48] are the most major compartments of the AF’s scaffold biochemical studies. Major factors governing biocompatibility and immunogenicity of the AF scaffolds’ biomaterials including molecular aspect (Size and shape), architectural properties (morphology, orientation, surface topography, porosity), surface chemistry and implantation site have been extensively overlooked through many studies as they are in common with other tissue engineering applications [127]. Therefore adaptive and innate immune response of silk biomaterials in different conditions and environment were explored [120, 121, 127].
In-vitro culture of human nasal chondrocytes on engineered fibers with alternating angled orientation demonstrated cell alignment with fibers that showed deposition of an orientation collagenous matrix, mimicking the organization and arrangement of native AF [60]. It was discussed that a scaffold with proper action have to able to produced extracellular matrix rich in GAG and collagen after implantation [25]. The influence of boundary geometry and composition of scaffold containing collagen gel on cell alignment was studied and shown that in the regions where cells were in tight proximity, collagen fibers were rearranged to form larger bundles on lines between cells. The more concentration of collagen with in the injectable scaffold, the more development of circumferential collagen fibril and cellular alignment [87].
Biochemical and histological analysis shown that coupling the silk scaffold with peptides affected cell morphology, however; no contribution was seen in cell formation and attachment as the surface treatment seemed to reduce scaffold porosity’s size and amount where porous silk is an appropriate scaffold on which to grow the AF cells [62].
Improvement of tissue formation probability within the silk scaffold in different cell culture methods (dynamic and static) along with their effect on cell diffusion into larger pore size of scaffold had been studied. Significantly more matrix generation in dynamic cell culture with specific scaffold’s pore size (600 µm pore diameter demonstrated more cell uniformity distribution and greatest amount of collagen I) result in better AF tissue formation and uniform spatial cell distribution [73, 93].
Insoluble cross-linked membranes of chitosan nanofibers diameter with the range of 150 – 650 nm were investigated to be very similar to the AF’s natural ECM both in composition and structure and exhibited excellent biocompatibility property and proper degradation rate, in vivo [144]. It was shown that in situ photo chemically crosslinking of collagen within the injectable scaffold survived physiologically relevant compression and torsion loading. Also it was proved that effectively reduced leakage and osteophyte formation in an animal study [88].
In some studies relation between development of the AF cells with simultaneous mechanical loading during cell culture process studied and it was shown that various bilateral tensile strain duration need to be optimized in order to lead cell-matrix interaction enhancement for IVD tissue engineering [13, 48, 103].
Table 3 indicates biochemical analysis related equipment or different assays usually had been used in different research activities.
Table 3.
Protocols, assays and equipment in the annulus biochemical evaluation activities based on scaffold engineering
| Equipment | Studied item and references |
|---|---|
| Scanning electron microscopy (SEM) | Scaffold morphology [20, 51, 53, 68, 82, 127, 134, 136, 139, 140], pore size measurement [81, 136], cell morphology [82, 97, 127] |
| Atomic force microscopy (AFM) | |
| Transmission electron microscopy (TEM) | |
| Contact angle measurement | Hydrophobicity [139] |
| β-Scintillation counter (β-liquid scintillation counter) | Collagen synthesis quantification [51, 70, 106], proteoglycan quantification [70, 106], GAG content analysis [49] |
| Confocal microscopy imaging follow by staining | Collagen and fibronectin synthesis and organization by AF cells [51], histology [27, 60], cell appearance and viability [86, 140] |
| Second harmonic generation (SHG) and two-photon excited fluorescence (TPEF) microscopy | Cell—scaffold interaction imaging [94] |
| Spinner flask (bioreactor) | Dynamic AF cell culture [80, 100] |
| ATR-FTIR analysis | Scaffold bonding and content [35, 86, 124, 127, 140] |
| Assays, protocols and standards | Studied item and references |
|---|---|
| Analysis of pro-inflammatory signal transduction pathways | Inflammation [139] |
| Quantitative ELISA assays | Cytokine release analysis [139], Silk binding assays [137] |
| Quantitative real time PCR | Gene expression [139] |
| Dye binding (Hoechst 33,258) assay followed by fluorometric analysis | Annulus fibrosis cell attachment [51], DNA content of the tissues determination [53, 80, 81, 83] |
| Live-dead assay | AF cell viability [81, 86, 127, 138, 140] |
| Dimethylmethylene blue assay | Glycosaminoglycan content [17, 53, 70, 83], proteoglycan content [53, 70] |
Conclusion
Introducing advanced technologies that developed methods to characterize and measure different properties of the AF in multi-scale levels, provided the opportunities to improve current understanding and addressed unresolved research questions. Research in tissue engineering of the AF has grown rapidly during the past decade. Nonetheless, one main unsolved point is about the role of the inter-lamellar space between adjacent lamellae, for which the question “how should the reconstructed lamellae be interconnected?” remains still unanswered. Unfortunately, the researcher in the area have not considered the ILM’s impact on the integrity of the AF structure. It was shown that this potentially important subject hadn’t put into consideration neither in selecting biomaterials and fabrication method nor in scaffold evaluation process.
On the other hand using hydrogels confined to the NP reconstruction or substitution rather than the AF tissue engineering. However selecting hydrogels with proper mechanical properties as the AF scaffold may result in preventing tissue dehydration that postpone degeneration process.
It seems that in spite of successful development in fabrication methods, more progress have to be achieved to help researchers make integrated scaffold with the properties that mimic the AF inter-connectivity. Further research is needed to introduce scaffolds that mimic the compositional structure and viscoelastic properties of the AF.
Conflict of interest
The author has no commercial relationship that may lead to a conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Choi YS. Pathophysiology of degenerative disc disease. Asian Spine J. 2009;3:39–44. doi: 10.4184/asj.2009.3.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerin HA, Elliott DM. Degeneration affects the fiber reorientation of human annulus fibrosus under tensile load. J Biomech. 2006;39:1410–1418. doi: 10.1016/j.jbiomech.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwale F, Ciobanu I, Giannitsios D, Roughley P, Steffen T, Antoniou J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine (Phila Pa 1976) 2011;36:E131–E138. doi: 10.1097/BRS.0b013e3181d52b9e. [DOI] [PubMed] [Google Scholar]
- 5.Sivan SS, Hayes AJ, Wachtel E, et al. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur Spine J. 2014;23:S344–S353. doi: 10.1007/s00586-013-2767-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Q, Gao X, Gu W. Temporal changes of mechanical signals and extracellular composition in human intervertebral disc during degenerative progression. J Biomech. 2014;47:3734–3743. doi: 10.1016/j.jbiomech.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalek AJ, Buckley MR, Bonassar LJ, Cohen I, Iatridis JC. The effects of needle puncture injury on microscale shear strain in the intervertebral disc annulus fibrosus. Spine J. 2010;10:1098–1105. doi: 10.1016/j.spinee.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade KR, Robertson PA, Thambyah A, Broom ND. How healthy discs herniate: a biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine (Phila Pa 1976) 2014;39:1018–1028. doi: 10.1097/BRS.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 9.Cortes DH, Jacobs NT, DeLucca JF, Elliott DM. Elastic, permeability and swelling properties of human intervertebral disc tissues: a benchmark for tissue engineering. J Biomech. 2014;47:2088–2094. doi: 10.1016/j.jbiomech.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CY, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184–1196. doi: 10.1016/j.jbiomech.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson AR, Yuan TY, Huang CY, Brown MD, Gu WY. Nutrient transport in human annulus fibrosus is affected by compressive strain and anisotropy. Ann Biomed Eng. 2012;40:2551–2558. doi: 10.1007/s10439-012-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson AR, Yuan TY, Huang CY, Travascio F, Yong GuW. Effect of compression and anisotropy on the diffusion of glucose in annulus fibrosus. Spine (Phila Pa 1976) 2008;33:1–7. doi: 10.1097/BRS.0b013e31815e4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes DH, Han WM, Smith LJ, Elliott DM. Mechanical properties of the extra-fibrillar matrix of human annulus fibrosus are location and age dependent. J Orthop Res. 2013;31:1725–1732. doi: 10.1002/jor.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desrochers J, Duncan NA. Strain transfer in the annulus fibrosus under applied flexion. J Biomech. 2010;43:2141–2148. doi: 10.1016/j.jbiomech.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 15.Han WM, Nerurkar NL, Smith LJ, Jacobs NT, Mauck RL, Elliott DM. Multi-scale structural and tensile mechanical response of annulus fibrosus to osmotic loading. Ann Biomed Eng. 2012;40:1610–1621. doi: 10.1007/s10439-012-0525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingsworth NT, Wagner DR. The stress and strain states of the posterior annulus under flexion. Spine (Phila Pa 1976) 2012;37:E1134–E1139. doi: 10.1097/BRS.0b013e318259aa60. [DOI] [PubMed] [Google Scholar]
- 17.Iatridis JC, ap Gwynn I. Mechanisms for mechanical damage in the intervertebral disc annulus fibrosus. J Biomech. 2004;37:1165–1175. doi: 10.1016/j.jbiomech.2003.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaacs JL, Vresilovic E, Sarkar S, Marcolongo M. Role of biomolecules on annulus fibrosus micromechanics: effect of enzymatic digestion on elastic and failure properties. J Mech Behav Biomed Mater. 2014;40:75–84. doi: 10.1016/j.jmbbm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27:800–806. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis NT, Hussain MA, Mao JJ. Investigation of nano-mechanical properties of annulus fibrosus using atomic force microscopy. Micron. 2008;39:1008–1019. doi: 10.1016/j.micron.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. Complex loading affects intervertebral disc mechanics and biology. Osteoarthr Cartil. 2011;19:1011–1018. doi: 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bron JL, Helder MN, Meisel HJ, Van Royen BJ, Smit TH. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–313. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho H, Park SH, Park K, et al. Construction of a tissue-engineered annulus fibrosus. Artif Organs. 2013;37:E131–E138. doi: 10.1111/aor.12066. [DOI] [PubMed] [Google Scholar]
- 25.Bowles RD, Gebhard HH, Hartl R, Bonassar LJ. Tissue-engineered intervertebral discs produce new matrix, maintain disc height, and restore biomechanical function to the rodent spine. Proc Natl Acad Sci U S A. 2011;108:13106–13111. doi: 10.1073/pnas.1107094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costi JJ, Stokes IA, Gardner-Morse M, Laible JP, Scoffone HM, Iatridis JC. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. J Biomech. 2007;40:2457–2466. doi: 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guterl CC, See EY, Blanquer SB, et al. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cells Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tampier C, Drake JDM, Callaghan JP, McGill SM. Progressive disc herniation—an investigation of the mechanism using radiologic, histochemical, and microscopic dissection techniques on a porcine model. Spine. 2007;32:2869–2874. doi: 10.1097/BRS.0b013e31815b64f5. [DOI] [PubMed] [Google Scholar]
- 29.Ciapetti G, Granchi D, Devescovi V, et al. Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J. 2012;21:S10–S19. doi: 10.1007/s00586-012-2234-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory DE, Bae WC, Sah RL, Masuda K. Anular delamination strength of human lumbar intervertebral disc. Eur Spine J. 2012;21:1716–1723. doi: 10.1007/s00586-012-2308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–262. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. J Anat. 2006;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veres SP, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus part II: how the annulus fails under hydrostatic pressure. Spine. 2008;33:2711–2720. doi: 10.1097/BRS.0b013e31817bb906. [DOI] [PubMed] [Google Scholar]
- 34.Roughley PJ. Biology of intervertebral disc aging and degeneration - Involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 35.Gregory DE, Bae WC, Sah RL, Masuda K. Disc degeneration reduces the delamination strength of the annulus fibrosus in the rabbit annular disc puncture model. Spine J. 2014;14:1265–1271. doi: 10.1016/j.spinee.2013.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharifi S, Bulstra SK, Grijpma DW, Kuijer R. Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J Tissue Eng Regen Med. 2015;9:1120–32. [DOI] [PubMed]
- 37.Nerurkar NL, Mauck RL, Elliott DM. ISSLS prize winner: integrating theoretical and experimental methods for functional tissue engineering of the annulus fibrosus. Spine (Phila Pa 1976) 2008;33:2691–2701. doi: 10.1097/BRS.0b013e31818e61f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Hudson KD, Alimi M, Grunert P, Hartl R, Bonassar LJ. Recent advances in biological therapies for disc degeneration: tissue engineering of the annulus fibrosus, nucleus pulposus and whole intervertebral discs. Curr Opin Biotechnol. 2013;24:872–879. doi: 10.1016/j.copbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Lazebnik M, Singh M, Glatt P, Friis LA, Berkland CJ, Detamore MS. Biomimetic method for combining the nucleus pulposus and annulus fibrosus for intervertebral disc tissue engineering. J Tissue Eng Regen Med. 2011;5:e179–e187. doi: 10.1002/term.412. [DOI] [PubMed] [Google Scholar]
- 41.Attia M, Santerre JP, Kandel RA. The response of annulus fibrosus cell to fibronectin-coated nanofibrous polyurethane-anionic dihydroxyoligomer scaffolds. Biomaterials. 2011;32:450–460. doi: 10.1016/j.biomaterials.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Barker IA, Ablett MP, Gilbert HTJ, et al. A microstereolithography resin based on thiol-ene chemistry: towards biocompatible 3D extracellular constructs for tissue engineering. Biomater Sci. 2014;2:472–475. doi: 10.1039/c3bm60290g. [DOI] [PubMed] [Google Scholar]
- 43.Jeong CG, Francisco AT, Niu Z, Mancino RL, Craig SL, Setton LA. Screening of hyaluronic acid-poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomater. 2014;10:3421–3430. doi: 10.1016/j.actbio.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barreto Henriksson H, et al. Similar cellular migration patterns from niches in intervertebral disc and in knee-joint regions detected by in situ labeling: an experimental study in the New Zealand white rabbit. Stem Cell Res Ther. 2013;4:104. doi: 10.1186/scrt315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruehlmann SB, Hulme PA, Duncan NA. In situ intercellular mechanics of the bovine outer annulus fibrosus subjected to biaxial strains. J Biomech. 2004;37:223–231. doi: 10.1016/s0021-9290(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 46.Chan SCW, Gantenbein-Ritter B. Preparation of intact bovine tail intervertebral discs for organ culture. J Vis Exp. 2012;60:e3490. doi: 10.3791/3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan WC, et al. Coming together is a beginning: the making of an intervertebral disc. Birth Defects Res C Embryo Today. 2014;102:83–100. doi: 10.1002/bdrc.21061. [DOI] [PubMed] [Google Scholar]
- 48.Clouet J, Grimandi G, Pot-Vaucel M, et al. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology. 2009;48:1447–1450. doi: 10.1093/rheumatology/kep262. [DOI] [PubMed] [Google Scholar]
- 49.Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL-1β sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J Cell Biochem. 2001;82:290–298. doi: 10.1002/jcb.1153. [DOI] [PubMed] [Google Scholar]
- 50.Feng G, Li L, Liu H, et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthr Cartil. 2013;21:582–588. doi: 10.1016/j.joca.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Gonzales S, Rodriguez B, Barrera C, Huang CY. Measurement of ATP-induced membrane potential changes in IVD cells. Cell Mol Bioeng. 2014;7:598–606. doi: 10.1007/s12195-014-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzales S, Wang C, Levene H, Cheung HS, Huang CY. ATP promotes extracellular matrix biosynthesis of intervertebral disc cells. Cell Tissue Res. 2015;359:635–642. doi: 10.1007/s00441-014-2042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gruber HE, Leslie K, Ingram J, Norton HJ, Hanley EN. Cell-based tissue engineering for the intervertebral disc: in vitro studies of human disc cell gene expression and matrix production within selected cell carriers. Spine J. 2004;4:44–55. doi: 10.1016/s1529-9430(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 54.Hegewald AA, Medved F, Feng D, et al. Enhancing tissue repair in annulus fibrosus defects of the intervertebral disc: analysis of a bio-integrative annulus implant in an in vivo ovine model. J Tissue Eng Regen Med. 2015;9:405–414. doi: 10.1002/term.1831. [DOI] [PubMed] [Google Scholar]
- 55.Hsieh AH, Twomey JD. Cellular mechanobiology of the intervertebral disc: new directions and approaches. J Biomech. 2010;43:137–145. doi: 10.1016/j.jbiomech.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Liu C, Guo Q, Yang H, Li B. Regional variations in the cellular, biochemical, and biomechanical characteristics of rabbit annulus fibrosus. PLoS ONE. 2014;9:e91799. doi: 10.1371/journal.pone.0091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu C, Guo Q, Li J, et al. Identification of rabbit annulus fibrosus-derived stem cells. PLoS ONE. 2014;9:e108239. doi: 10.1371/journal.pone.0108239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moon HJ, Yurube T, Lozito TP, et al. Effects of secreted factors in culture medium of annulus fibrosus cells on microvascular endothelial cells: elucidating the possible pathomechanisms of matrix degradation and nerve in-growth in disc degeneration. Osteoarthr Cartil. 2014;22:344–354. doi: 10.1016/j.joca.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu H, Xu B, Yang Q, et al. Comparison of decellularization protocols for preparing a decellularized porcine annulus fibrosus scaffold. PLoS ONE. 2014;9:e86723. doi: 10.1371/journal.pone.0086723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhattacharjee M, Miot S, Gorecka A, et al. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: towards annulus fibrosus tissue engineering. Acta Biomater. 2012;8:3313–3325. doi: 10.1016/j.actbio.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 61.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue specific considerations. Eur Spine J. 2008;17:S467–S479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang G, Kim HJ, Kaplan D, Vunjak-Novakovic G, Kandel RA. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur Spine J. 2007;16:1848–1857. doi: 10.1007/s00586-007-0364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du LL, Zhu MF, Yang Q, et al. A novel integrated biphasic silk fibroin scaffold for intervertebral disc tissue engineering. Mater Lett. 2014;15:237–240. [Google Scholar]
- 64.Ha HJ, Kim SH, Yoon SJ, et al. Evaluation of various scaffolds for tissue engineered biodisc using annulus fibrosus cells. Polym Korea. 2008;32:26–30. [Google Scholar]
- 65.Martin JT, Milby AH, Chiaro JA, et al. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014;10:2473–2481. doi: 10.1016/j.actbio.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saad L, Spector M. Effects of collagen type on the behavior of adult canine annulus fibrosus cells in collagen-glycosaminoglycan scaffolds. J Biomed Mater Res A. 2004;71:233–241. doi: 10.1002/jbm.a.30150. [DOI] [PubMed] [Google Scholar]
- 67.Vadala G, Mozetic P, Rainer A, et al. Bioactive electrospun scaffold for annulus fibrosus repair and regeneration. Eur Spine J. 2012;21:S20–S26. doi: 10.1007/s00586-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan Y, Feng G, Shen FH, Balian G, Laurencin CT, Li X. Novel biodegradable poly(1,8-octanediol malate) for annulus fibrosus regeneration. Macromol Biosci. 2007;7:1217–1224. doi: 10.1002/mabi.200700053. [DOI] [PubMed] [Google Scholar]
- 69.Wan Y, Feng G, Shen FH, Laurencin CT, Li X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials. 2008;29:643–652. doi: 10.1016/j.biomaterials.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Wismer N, Grad S, Fortunato G, Ferguson SJ, Alini M, Eglin D. Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Eng Part A. 2014;20:672–682. doi: 10.1089/ten.TEA.2012.0679. [DOI] [PubMed] [Google Scholar]
- 71.Wu YH, Xu BS, Yang Q, et al. A novel natural ECM-derived biphasic scaffold for intervertebral disc tissue engineering. Mater Lett. 2013;105:102–105. [Google Scholar]
- 72.Busby GA, Grant MH, MacKay SP, Riches PE. Confined compression of collagen hydrogels. J Biomech. 2013;46:837–840. doi: 10.1016/j.jbiomech.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 73.Chang G, Kim HJ, Vunjak-Novakovic G, Kaplan DL, Kandel R. Enhancing annulus fibrosus tissue formation in porous silk scaffolds. J Biomed Mater Res A. 2010;92:43–51. doi: 10.1002/jbm.a.32326. [DOI] [PubMed] [Google Scholar]
- 74.Guillaume O, Daly A, Lennon K, Gansau J, Buckley SF, Buckley CT. Shape-memory porous alginate scaffolds for regeneration of the annulus fibrosus: effect of TGF-beta3 supplementation and oxygen culture conditions. Acta Biomater. 2014;10:1985–1995. doi: 10.1016/j.actbio.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 75.Helen W, Gough JE. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass composite foam scaffolds in vitro. Acta Biomater. 2008;4:230–243. doi: 10.1016/j.actbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Koepsell L, Remund T, Bao J, Neufeld D, Fong H, Deng Y. Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. J Biomed Mater Res A. 2011;99:564–575. doi: 10.1002/jbm.a.33216. [DOI] [PubMed] [Google Scholar]
- 77.Nesti LJ, Li WJ, Shanti RM, et al. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng Part A. 2008;14:1527–1537. doi: 10.1089/ten.tea.2008.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park SH, Gil ES, Cho H, et al. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng Part A. 2012;18:447–458. doi: 10.1089/ten.tea.2011.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SH, Gil ES, Mandal BB, et al. Annulus fibrosus tissue engineering using lamellar silk scaffolds. J Tissue Eng Regen Med. 2012;6:s24–s33. doi: 10.1002/term.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pirvu T, Blanquer SB, Benneker LM, et al. A combined biomaterial and cellular approach for annulus fibrosus rupture repair. Biomaterials. 2015;42:11–19. doi: 10.1016/j.biomaterials.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 81.Sha’ban M, Yoon SJ, Ko YK, et al. Fibrin promotes proliferation and matrix production of intervertebral disc cells cultured in three-dimensional poly(lactic-co-glycolic acid) scaffold. J Biomater Sci Polym Ed. 2008;19:1219–1237. doi: 10.1163/156856208785540163. [DOI] [PubMed] [Google Scholar]
- 82.Shao X, Hunter CJ. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res A. 2007;82:701–710. doi: 10.1002/jbm.a.31030. [DOI] [PubMed] [Google Scholar]
- 83.Song JE, Kim EY, Ahn WY, et al. The potential of DBP gels containing intervertebral disc cells for annulus fibrosus supplementation: in vivo. J Tissue Eng Regen Med. 2015;9:E98–107. [DOI] [PubMed]
- 84.Turner KG, Ahmed N, Santerre JP, Kandel RA. Modulation of annulus fibrosus cell alignment and function on oriented nanofibrous polyurethane scaffolds under tension. Spine J. 2014;14:424–434. doi: 10.1016/j.spinee.2013.08.047. [DOI] [PubMed] [Google Scholar]
- 85.Wilda H, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglass composite films. Biomaterials. 2006;27:5220–5229. doi: 10.1016/j.biomaterials.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Xu B, Xu H, Wu Y, et al. Intervertebral disc tissue engineering with natural extracellular matrix-derived biphasic composite scaffolds. PLoS ONE. 2015;10:e0124774. doi: 10.1371/journal.pone.0124774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowles RD, Williams RM, Zipfel WR, Bonassar LJ. Self-assembly of aligned tissue engineered annulus fibrosus and intervertebral disc composite via collagen gel contraction. Tissue Eng Part A. 2010;16:1339–1348. doi: 10.1089/ten.tea.2009.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chik TK, Ma XY, Choy TH, et al. Photochemically crosslinked collagen annulus plug: a potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 2013;9:8128–8139. doi: 10.1016/j.actbio.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 89.Grunert P, Borde BH, Hudson KD, Macielak MR, Bonassar LJ, Hartl R. Annular repair using high-density collagen gel: a rat-tail in vivo model. Spine (Phila Pa 1976) 2014;39:198–206. doi: 10.1097/BRS.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guillaume O, Naqvi SM, Lennon K, Buckley CT. Enhancing cell migration in shape-memory alginate-collagen composite scaffolds: in vitro and ex vivo assessment for intervertebral disc repair. J Biomater Appl. 2015;29:1230–1246. doi: 10.1177/0885328214557905. [DOI] [PubMed] [Google Scholar]
- 91.Koepsell L, Zhang L, Neufeld D, Fong H, Deng Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol Biosci. 2011;11:391–399. doi: 10.1002/mabi.201000352. [DOI] [PubMed] [Google Scholar]
- 92.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986–992. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nerurkar NL, Sen S, Baker BM, Elliott DM, Mauck RL. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011;7:485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Revell PA, Damien E, Di SilvioL, Gurav N, Longinotti C, Ambrosio L. Tissue engineered intervertebral disc repair in the pig using injectable polymers. J Mater Sci Mater Med. 2007;18:303–308. doi: 10.1007/s10856-006-0693-6. [DOI] [PubMed] [Google Scholar]
- 95.Schek RM, Michalek AJ, Iatridis JC. Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. Eur Cell Mater. 2011;21:373–383. doi: 10.22203/ecm.v021a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharifi S, van Kooten TG, Kranenburg HJ, et al. An annulus fibrosus closure device based on a biodegradable shape-memory polymer network. Biomaterials. 2013;34:8105–8113. doi: 10.1016/j.biomaterials.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 97.Yeganegi M, Kandel RA, Santerre JP. Characterization of a biodegradable electrospun polyurethane nanofiber scaffold: mechanical properties and cytotoxicity. Acta Biomater. 2010;6:3847–3855. doi: 10.1016/j.actbio.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Chik TK, Chooi WH, Li YY, et al. Bioengineering a multicomponent spinal motion segment construct—a 3D model for complex tissue engineering. Adv Healthc Mater. 2015;4:99–112. doi: 10.1002/adhm.201400192. [DOI] [PubMed] [Google Scholar]
- 99.Driscoll TP, Nakasone RH, Szczesny SE, Elliott DM, Mauck RL. Biaxial mechanics and interlamellar shearing of stem-cell seeded electrospun angle-ply laminates for annulus fibrosus tissue engineering. J Orthop Res. 2013;31:864–870. doi: 10.1002/jor.22312. [DOI] [PubMed] [Google Scholar]
- 100.Driscoll TP, Nerurkar NL, Jacobs NT, Elliott DM, Mauck RL. Fiber angle and aspect ratio influence the shear mechanics of oriented electrospun nanofibrous scaffolds. J Mech Behav Biomed Mater. 2011;4:1627–1636. doi: 10.1016/j.jmbbm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borde B, Grunert P, Hartl R, Bonassar LJ. Injectable, high-density collagen gels for annulus fibrosus repair: an in vitro rat tail model. J Biomed Mater Res A 2014;11. [DOI] [PMC free article] [PubMed]
- 102.Bron JL, van der Veen AJ, Helder MN, et al. Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. Eur Spine J. 2010;19:1347–1355. doi: 10.1007/s00586-010-1384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chuah YJ, Lee WC, Wong HK, Kang Y, Hee HT. Three-dimensional development of tensile pre-strained annulus fibrosus cells for tissue regeneration: an in vitro study. Exp Cell Res. 2015;331:176–182. doi: 10.1016/j.yexcr.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 104.Mizuno H, Roy AK, Zaporojan V, Vacanti CA, Ueda M, Bonassar LJ. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials. 2006;27:362–370. doi: 10.1016/j.biomaterials.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 105.Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–1028. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- 106.Blanquer SB, Grijpma DW, Poot AA. Delivery systems for the treatment of degenerated intervertebral discs. Adv Drug Deliv Rev. 2015;84:172–187. doi: 10.1016/j.addr.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 107.Pritchard EM, Valentin T, Boison D, Kaplan DL. Incorporation of proteinase inhibitors into silk-based delivery devices for enhanced control of degradation and drug release. Biomaterials. 2011;32:909–918. doi: 10.1016/j.biomaterials.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan SCW, Gantenbein-Ritter B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy-feasible or fiction? Swiss Med Wkly 31 2012;142. [DOI] [PubMed]
- 109.Gebhard H, Bowles R, Dyke J, et al. Total disc replacement using a tissue-engineered intervertebral disc in vivo: new animal model and initial results. Evid Based spine-Care J. 2010;1:62–66. doi: 10.1055/s-0028-1100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hiyama A, Mochida J, Sakai D. Stem cell applications in intervertebral disc repair. Cell Mol Biol. 2008;54:24–32. [PubMed] [Google Scholar]
- 111.Jin L, Shimmer AL, Li X. The challenge and advancement of annulus fibrosus tissue engineering. Eur Spine J. 2013;22:1090–1100. doi: 10.1007/s00586-013-2663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kalson NS, Richardson S, Hoyland JA. Strategies for regeneration of the intervertebral disc. Regen Med. 2008;3:717–729. doi: 10.2217/17460751.3.5.717. [DOI] [PubMed] [Google Scholar]
- 113.Kandel R, Roberts S, Urban JPG. Tissue engineering and the intervertebral disc: the challenges. Eur Spine J. 2008;17:S480–S491. doi: 10.1007/s00586-008-0746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16:147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silva-Correia J, Correia SI, Oliveira JM, Reis RL. Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnol Adv. 2013;31:1514–1531. doi: 10.1016/j.biotechadv.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 116.Blanquer SBG, Sharifi S, Grijpma DW. Development of poly(trimethylene carbonate) network implants for annulus fibrosus tissue engineering. J Appl Biomater Funct. 2012;10:177–184. doi: 10.5301/JABFM.2012.10354. [DOI] [PubMed] [Google Scholar]
- 117.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 119.Kang H-W, Seol Y-J, Cho D-W. Development of an indirect solid freeform fabrication process based on microstereolithography for 3D porous scaffolds. J Micromech Microeng. 2009;19:015011. [Google Scholar]
- 120.Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 121.Calabrese R, Kaplan DL. Silk ionomers for encapsulation and differentiation of human MSCs. Biomaterials. 2012;33:7375–7385. doi: 10.1016/j.biomaterials.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 123.Numata K, Cebe P, Kaplan DL. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials. 2010;31:2926–2933. doi: 10.1016/j.biomaterials.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–9224. doi: 10.1016/j.biomaterials.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.An B, DesRochers TM, Qin GK, et al. The influence of specific binding of collagen-silk chimeras to silk biomaterials on hMSC behavior. Biomaterials. 2013;34:402–412. doi: 10.1016/j.biomaterials.2012.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773–5781. doi: 10.1016/j.biomaterials.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhattacharjee M, Schultz-Thater E, Trella E, et al. The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials. Biomaterials. 2013;34:8161–8171. doi: 10.1016/j.biomaterials.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 128.Lu QA, Wang XL, Lu SZ, Li MZ, Kaplan DL, Zhu HS. Nanofibrous architecture of silk fibroin scaffolds prepared with a mild self-assembly process. Biomaterials. 2011;32:1059–1067. doi: 10.1016/j.biomaterials.2010.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang YZ, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 130.Zhang YF, Wu CT, Friis T, Xiao Y. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials. 2010;31:2848–2856. doi: 10.1016/j.biomaterials.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 131.Blaker JJ, Maquet V, Jerome R, Boccaccini AR, Nazhat SN. Mechanical properties of highly porous PDLLA/Bioglass (R) composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005;1:643–652. doi: 10.1016/j.actbio.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Maquet V, Boccaccini AR, Pravata L, Notingher I, Jerome R. Porous poly(alphahydroxyacid)/Bioglass (R) composite scaffolds for bone tissue engineering. I: preparation and in vitro characterisation. Biomaterials. 2004;25:4185–4194. doi: 10.1016/j.biomaterials.2003.10.082. [DOI] [PubMed] [Google Scholar]
- 133.Li D, Wang YL, Xia YN. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003;3:1167–1171. [Google Scholar]
- 134.Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: From classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–7470. doi: 10.1016/j.biomaterials.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 135.Soares RR, Carone C, Einloft S, Ligabue R, Monteiro WF. Synthesis and characterization of waterborne polyurethane/ZnO composites. Polym Bull. 2014;71:829–838. [Google Scholar]
- 136.Fernandez-d’Arlas B, Eceiza A. Functionalization of multiwalled carbon nanotubes with urethane segments and their interaction with solvents and a polyurethane elastomer. J Nanopart Res. 2013;16:1–10. [Google Scholar]
- 137.Stokes K, Mcvenes R, Anderson JM. Polyurethane elastomer biostability. J Biomater Appl. 1995;9:321–354. doi: 10.1177/088532829500900402. [DOI] [PubMed] [Google Scholar]
- 138.Smith R, Oliver C, Williams DF. The enzymatic degradation of polymers in vitro. J Biomed Mater Res. 1987;21:991–1003. doi: 10.1002/jbm.820210805. [DOI] [PubMed] [Google Scholar]
- 139.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 140.Yang L. Polar surface chemistry of nanofibrous polyurethane scaffold affects annulus fibrosus cell attachment and early matrix accumulation. J Biomed Mater Res, Part A. 2009;91A:1089–1099. doi: 10.1002/jbm.a.32331. [DOI] [PubMed] [Google Scholar]
- 141.Spiteri C, Raizman I, Pilliar RM, Kande RA. Matrix accumulation by articular chondrocytes during mechanical stimulation is influenced by integrin-mediated cell spreading. J Biomed Mater Res A. 2010;94A:122–129. doi: 10.1002/jbm.a.32706. [DOI] [PubMed] [Google Scholar]
- 142.Huang C, et al. Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf B Biointerfaces. 2011;82:307–315. doi: 10.1016/j.colsurfb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 143.Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54:626–632. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen L, et al. A human-like collagen/chitosan electrospun nanofibrous scaffold from aqueous solution: electrospun mechanism and biocompatibility. J Biomed Mater Res, Part A. 2011;99A:395–409. doi: 10.1002/jbm.a.33202. [DOI] [PubMed] [Google Scholar]
- 145.Zhu CH, Fan DD, Duan ZZ, et al. Initial investigation of novel human-like collagen/chitosan scaffold for vascular tissue engineering. J Biomed Mater Res A. 2009;89A:829–840. doi: 10.1002/jbm.a.32256. [DOI] [PubMed] [Google Scholar]
- 146.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 147.Guan JJ, Gao CY, Feng LX, Shen JC. Functionalizing of polyurethane surfaces by photo grafting with hydrophilic monomers. J Appl Polym Sci. 2000;77:2505–2512. [Google Scholar]
- 148.Stankus JJ, Guan JJ, Wagner WR. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res A. 2004;70A:603–614. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mi HY, Salick MR, Jing X, Crone WC, Peng XF, Turng LS. Electrospinning of uni-directionally and orthogonally aligned thermoplastic polyurethane nanofibers: Fiber orientation and cell migration. J Biomed Mater Res A. 2015;103:593–603. doi: 10.1002/jbm.a.35208. [DOI] [PMC free article] [PubMed] [Google Scholar]