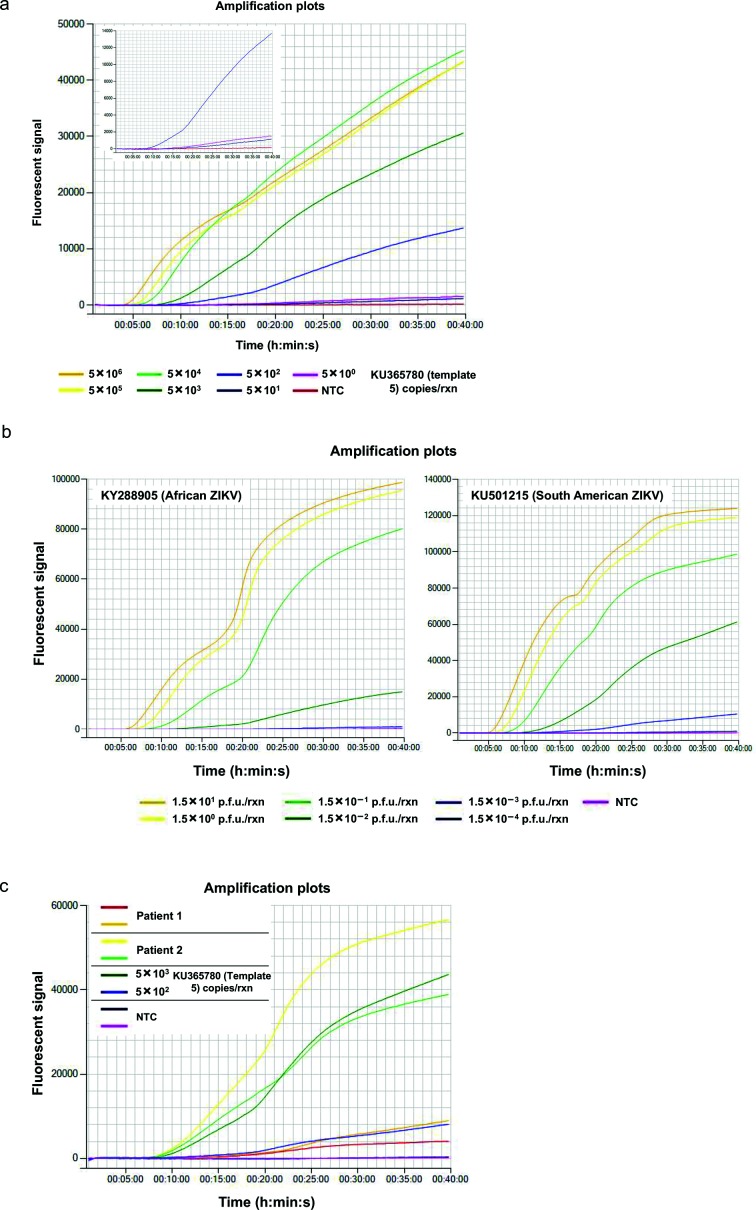
Fig. 5.
Performance of the ZIKV RT-RPA assay on a portable battery-operated instrument, the Genie III by OptiGene. (a) Tenfold dilution series of Zika synthetic RNA template 5 (KU365780) used to determine the lowest number of target molecules detected by the ZIKV RT-RPA assay. The insert displays the fluorescent signal generated from the amplification of 500, 50 and 5 copies of ZIKV synthetic RNA template 5 in comparison to the non-template control (NTC) in the ZIKV RT-RPA assay. (b) Tenfold dilution series of extracted nucleic acid from two strains of cultured ZIKV (African – KY288905, left panel, and South American – KU501215, right panel), as detected by the ZIKV RT-RPA assay and compared to the non-template control (NTC). (c) Detection of clinical samples from two patients in duplicates with low ZIKV titre (patient 1) and high ZIKV titre (patient 2) in comparison to 5×103 and 5×102 copies of Zika synthetic RNA template 5 (KU365780) and the non-template control (NTC) by the ZIKV RT-RPA.