Watch a video presentation of this article
Watch the interview with the author
Abbreviation
- MELD
Model for End‐Stage Liver Disease
It is easy to identify a patient with cirrhosis who needs liver transplantation. Governed by the patient's risk for mortality, the decision to list a cirrhotic patient for liver transplantation is based on the presence of overt manifestations of cirrhosis, such as ascites, hepatic encephalopathy, or sarcopenia, and a Model for End‐Stage Liver Disease (MELD) score ≥15.1 The specificity of these listing criteria, as outlined by the American Association for the Study of Liver Diseases and the American Transplantation Society Practice Guidelines,1 belies the dynamic nature of the decision to proceed with transplant surgery at the time that the patient is at the top of the list. Despite the emphasis on waitlist mortality risk when deciding to list a patient for liver transplant, the decision to proceed with the surgery is, in practice, weighted much more on the candidate's ability to survive after the operation than his or her risk for dying without it.
Little consensus exists in the literature on how a transplant clinician should approach this decision. As a result, this decision is made on a case‐by‐case approach based on little more than the physicians' clinical intuition of how the patient will fare in the immediate postoperative period. Although there is no more powerful tool in the practice of medicine than clinical judgment,2 lack of a guiding framework leaves us vulnerable to factors that are understandably difficult to separate from our decisions,3 but perhaps should not influence them. As an example, the frequent follow‐up necessitated by a cirrhotic patient's recurrent decompensating events allows transplant clinicians to develop strong bonds with their patients and caregivers. But our empathy can cloud our judgment on what is “best” for the patient; sometimes the reason a patient so desperately needs a liver transplant (e.g., cachexia from weekly large‐volume paracenteses, disability from refractory hepatic encephalopathy) is the very reason he or she is too sick to receive it.
How can we develop practice guidelines for a decision as dynamic and personal as when to say no to a transplant for our own patient on the waitlist? Rather than specific criteria, I propose a framework for individualized patient‐level decision making (Fig. 1).4 In this framework, the decision to proceed with liver transplant depends not on a patient's risk for waitlist mortality but, rather, the likelihood that a new liver will reverse the patient's pretransplant “vulnerabilities.” By vulnerability, I mean any condition that decreases physiological reserve and increases the risk for adverse health outcomes to any acute stressor. Examples of conditions that will reverse quickly after transplant, what I term transplant‐responsive conditions, include ascites and coagulopathy. Before transplant, ascites and coagulopathy significantly increase the risk for decompensation from spontaneous bacterial peritonitis or life‐threatening hemorrhage, respectively, which can be eliminated as early as postoperative day one (candidate A). Examples of conditions that will not reverse after liver transplant, that is, “transplant nonresponsive,” include triple‐vessel coronary artery disease, diabetic peripheral neuropathy, or gastroparesis. Conditions such as these not only threaten the long‐term success of liver transplant with respect to both survival and quality of life, but may even accelerate as a result of transplant and immunosuppression (candidate C).
Figure 1.
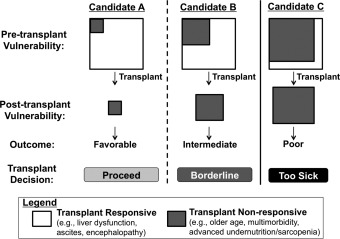
Framework for deciding when to say no to transplant for your listed patient. Adapted with permission from Current Opinion in Organ Transplant.4 Copyright 2016, Wolters Kluwer Health.
However, real‐life transplant decision making is not always as clear‐cut as for candidates A and C, who are on the extreme spectrums of transplant benefit. It is for patients like candidate B for whom a framework may hold its greatest utility (Fig. 1). An example of candidate B is a cirrhotic patient with refractory ascites for the last year who has experienced progressive muscle wasting, malnutrition, and functional decline. Although a new liver can cure portal hypertension seemingly instantly, it may not reverse frailty quickly enough for a patient to avoid the potentially lethal effects of postoperative immobility, opportunistic infections, and extrahepatic organ dysfunction (e.g., prolonged ventilator support, acute kidney injury). In contrast, with optimization of the transplant circumstances, such as with prehabilitation, early transplantation at a lower MELD score, and higher donor quality, candidate B may emerge from the peritransplant period to live a longer, better life.
Our challenge is to find ways to objectively quantify, and perhaps even improve, overall transplant benefit beyond survival alone, particularly for patients like candidate B for whom the benefit is less obvious. For this reason, I am proposing this framework not just for clinical decision making, but as a blueprint for future research (Table 1). This blueprint can be useful to identify methods to capture what may not reverse with liver transplant (moving vertically in Fig. 1). For example, objective measures of frailty, which predict pretransplant outcomes,5, 6, 7 are conceptually promising, but have yet to be shown to predict outcomes after transplant. In addition, we can use this framework to inspire research focused on shifting candidates leftward (i.e., from candidate C → B, or candidate B → A) with intensive prehabilitation or early transplantation before the patient develops irreversible complications of end‐stage liver disease or advanced comorbidities. Research that is focused on quantifying transplant benefit beyond survival alone will enable us to operationalize this framework for individualized patient‐level decision making.
Table 1.
Proposed Areas for Future Research Based on the Framework for Deciding When to Say No to Your Listed Patient
| Quantifying Transplant Benefit (moving vertically in Fig. 1) | Improving Transplant Benefit by Shifting Candidates From C → B or From B → A (moving horizontally in Fig. 1) |
| Pretransplant measures of frailty | Prehabilitation |
| Sarcopenia | Transplanting patients with greater transplant nonresponsive factors with higher donor quality |
| Biomarkers of frailty, sarcopenia, or renal dysfunction | “Early” transplant (i.e., at a lower MELD score) with living donor liver transplantation |
The Final Rule of the National Organ Transplant Act has long provided the transplant community with a regulatory framework for our organ allocation policies: when deciding who should undergo transplant, we must strive for a system that minimizes waitlist mortality while avoiding futility of transplantation.8 Although the incorporation of the MELD score into liver allocation has allowed us to achieve the first mandate (to minimize waitlist mortality), we have yet to systematize the concept of avoiding futility. Let us begin to use this clinical framework that I propose (Fig. 1) to help us define the circumstances in which we must say no—not just by federal mandate, but because the patient will not achieve the benefit from transplant that we would all want for ourselves and our loved ones. It is undoubtedly the most difficult decision that we as transplant clinicians have to make, and we owe it to our patients and their families to make this decision more objective, transparent, and uniform.
This study was supported by the UCSF Older Americans Independence Center (Grant P30AG044281), a Paul B. Beeson Career Development Award in Aging Research (K23AG048337), and the American Federation for Aging Research. These funding agencies played no role in the preparation of this editorial.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the AASLD and the American Society of Transplantation. Hepatology 2014:1‐98. [DOI] [PubMed] [Google Scholar]
- 2. Lai JC, Covinsky KE, Hayssen H, Lizaola B, Dodge JL, Roberts JP, et al. Clinician assessments of health status predict mortality in patients with end‐stage liver disease awaiting liver transplantation. Liver Int 2015;35:2167‐2173. doi: 10.1111/liv.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Volk ML, Biggins SW, Huang MA, Argo CK, Fontana RJ, Anspach RR. Decision making in liver transplant selection committees: a multicenter study. Ann Intern Med 2011;155:503‐508. doi: 10.7326/0003-4819-155-8-201110180-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai JC. Defining the threshold for too sick for transplant. Curr Opin Organ Transplant 2016;21:127‐132. doi: 10.1097/MOT.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870‐1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology 2016;63:574‐580. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol; doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 8. Organ Procurement and Transplantation Network . Final rule. https://optn.transplant.hrsa.gov/governance/about‐the‐optn/final‐rule/. Published March 16, 2000. Accessed September 20, 2016.


