Abstract
Lung cancer patients with human immunodeficiency virus (HIV) have a poorer prognosis than do patients without HIV infection. HIV1 Tat is a secreted viral protein that penetrates the plasma membrane and interacts with a number of proteins in non‐HIV‐infected cells. The loss of function of Tat‐interacting protein 30 (TIP30) has been linked to metastasis in non‐small cell lung cancer (NSCLC). However, it is unknown how the interaction of HIV1 Tat with TIP30 regulates the metastasis of NSCLC cells. In this study, the overexpression of TIP30 decreased tumor growth factor‐β‐induced epithelial‐to‐mesenchymal transition (EMT) and invasion of NSCLC cells, whereas the knockdown of TIP30 promoted EMT, invasion and stemness. Exposure to recombinant HIV1 Tat proteins promoted EMT and invasion. A mechanistic study showed that the interaction of HIV1 Tat with TIP30 blocked the binding of TIP30 to importin‐β, which is required for the nuclear translocation of Snail. Indeed, the loss of TIP30 promoted the nuclear translocation of Snail. In vivo studies demonstrated that the overexpression of TIP30 inhibited the metastasis of NSCLC cells. In contrast, the coexpression of HIV1 Tat and TIP30 diminished the inhibitory effect of TIP30 on metastasis. Immunohistochemistry confirmed that TIP30 overexpression reduced the nuclear localization of Snail, whereas the coexpression of HIV1 Tat and TIP30 increased nuclear Snail in metastatic tumors. In conclusion, the binding of HIV1 Tat to TIP30 enhanced EMT and metastasis by regulating the nuclear translocation of Snail. Targeting Tat‐interacting proteins may be a potential therapeutic strategy to prevent metastasis in NSCLC patients with HIV infection.
Keywords: epithelial‐to‐mesenchymal transition, non‐small cell lung cancer, nuclear translocation, Snail, Tat‐interacting protein
1. INTRODUCTION
Highly active antiretroviral therapy (HAART) significantly improves the survival and morbidity of patients with acquired immune deficiency syndrome (AIDS).1 Despite the effective suppression of the human immunodeficiency virus (HIV) viral load by HAART, the number of patients who live with HIV is increasing.2 In the modern HAART era, HIV infection is considered a chronic disease that causes cancers, thus bringing new health challenges to HIV‐infected patients. Among the non‐AIDS‐defining cancers, lung cancer is the most common malignancy; this phenomenon may be explained by several risk factors, such as smoking, immunosuppression and anti‐retroviral agent‐induced DNA damage.3 Lung cancer patients with HIV infection have poorer survival rates and prognoses compared with their HIV‐uninfected counterparts, although the molecular mechanism is not clear.4, 5
The Tat protein of HIV1 is a transcriptional activator that contributes to viral and endogenous gene expression in host cells.6 Even during effective HAART, the Tat protein is continuously released from HIV‐infected cells.7 The extracellular Tat protein may enter neighboring cells in its free form or through its interaction with membrane receptors.8 Although T cells are the major type of cells infected with HIV, the penetration of the Tat protein into different cell types is linked to several pathological events, including cancer.9, 10 These findings indicate that Tat‐mediated molecular mechanisms may play important roles in cancer progression.
Many Tat‐interacting molecules have been identified. Although Tat lacks a DNA‐binding domain, it interacts with several transcriptional regulators, such as RNA polymerase II, general transcription factors, and some additional transcription factors.11, 12 Tat‐interacting protein 30 (TIP30, also known as Htatip2) harbors intrinsic enzyme activities and functions as a positive regulator of HIV1 Tat‐activated transcription.13, 14, 15 TIP30 is considered to be a tumor suppressor in hepatocellular carcinoma (HCC) and colorectal carcinoma.16, 17 In addition, the downregulation of TIP30 is associated with poor prognosis and metastasis in different cancer types, including prostate cancer, gallbladder cancer, laryngeal squamous cell carcinoma, esophageal carcinoma and lung cancer,18, 19 although the underlying mechanism is not clear.
In addition to its function in transcriptional regulation, TIP30 deficiency results in the activation of the protein kinase B (Akt) signaling pathway, leading to proliferation, drug resistance and lipogenesis in laryngeal carcinoma and HCC.20, 21 The inhibition of TIP30 expression by microRNA‐10b enhances epidermal growth factor (EGF) and tumor growth factor (TGF)‐β signaling and contributes to the invasive ability of pancreatic cancer cells.22 In HCC, the loss of TIP30 promotes Snail‐mediated epithelial‐to‐mesenchymal transition (EMT), a biological process that promotes the migration, invasion and stemness of cancer cells.23 However, it is unclear how the interaction of Tat and TIP30 regulates metastasis in lung cancer. In this study, we showed that the HIV1 Tat protein promoted EMT and invasion in NSCLC cell lines. The forced expression of TIP30 reduced the TGF‐β‐induced EMT and invasion of NSCLC cells. In contrast, the knockdown of TIP30 or the binding of the HIV1 Tat protein to TIP30 enhanced TGF‐β‐induced EMT and invasion by promoting the nuclear translocation of Snail in NSCLC cells.
2. MATERIALS AND METHODS
2.1. Cell lines and cell culture
Human NSCLC cell lines (A549, H358, PC9 and PC13) and the human embryonic kidney 293T (HEK293T) cell line were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The A549 and HEK293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 100 U/mL penicillin, 100 U/mL streptomycin and 10% fetal bovine serum (FBS). The H358, PC9 and PC13 cell lines were cultured in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 U/mL streptomycin and 10% FBS. The cells were maintained in a humidified incubator at 37°C in a 5% CO2 atmosphere.
2.2. Plasmid construction, shRNA and lentivirus preparation
The oligonucleotides from His‐tagged human TIP30 (855 bp; NM_001098520.1), Flag‐tagged HIV1 Tat (336 bp; M64491.1) and human Snail (795 bp; NM_005985.3) were synthesized by Invitrogen (Carlsbad, CA, USA). The TIP30‐Flag DNA fragment was cloned into the pAS2w.Phyg lentiviral vector between the NheI and EcoRI restriction enzyme sites. The Flag‐tagged HIV1 Tat and Snail DNA fragments were individually cloned into the pLex‐MCS lentiviral vector between the BamHI and XhoI restriction enzyme sites.
The shRNA‐containing lentiviral vectors targeting human TIP30 were provided by the National RNAi Core Facility, Academia Sinica, Taipei, Taiwan. The identification numbers of the two shRNA clones used for the TIP30 knockdown were as follows: TRCN0000020350 (shTIP30 #1) and TRCN0000280399 (shTIP30 #2).
The lentiviral particles containing all the expression plasmids and shRNAs were prepared by co‐transfecting the psPAX2 and pMD2G plasmids into HEK293T cells. The virus‐containing medium was collected at 48 and 72 hours. The viral supernatant was centrifuged and filtered. Lentivirus was transduced into the indicated cell lines with polybrene (2 μg/mL). Stable clones of the individual infected cell lines were established by selection with puromycin or hygromycin.
2.3. TIP30 knockout by the CRISPR‐Cas9 genome editing system
A lentiviral vector, pAll‐Cas9.pPuro, containing the single guide RNA (sgRNA) targeting human TIP30, namely, sgTIP30, and the lentiviral vector containing the nontargeting control sgRNA, namely, sgControl, were purchased from the National RNAi Core Facility, Academia Sinica, Taipei, Taiwan. The sequence of TIP30‐targeting sgRNAs was as follows: sgTIP30, CGAAGCTTCGGGAAGACTTC. A549 cells were infected with lentivirus carrying the individual sgRNAs. The homozygous TIP30 knockout cells were selected by serial dilution and single‐cell culturing. The TIP30 knockout efficiency was confirmed by western blotting.
2.4. Animal study
All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. Age‐matched nude mice (6‐8 weeks old; male) were used. The mice were randomly divided into three groups as follows: (1) mice receiving a vector‐only H358 cell transplantation; (2) mice receiving a TIP30‐expressing H358 cell transplantation; and (3) mice receiving a TIP30‐His and HIV1 Tat‐Flag‐co‐expressing H358 cell transplantation. All transplanted cells were pre‐labeled with GFP and luciferase by a CMV promoter‐driven EGFP‐P2A‐Luciferase‐expressing lentivirus for the real‐time ex vivo detection of tumor growth. For the in vivo metastasis assays, 1 × 106 cells were resuspended in phosphate‐buffered saline and were orthotopically injected into the left lung of each mouse. Metastatic progression was measured with a noninvasive bioluminescence system (IVIS) at various time points. At the end of the experiment (<50 days), the mice were killed, and the lungs were harvested for further metastatic nodule quantification and histological analysis.
2.5. Immunohistochemistry (IHC)
Tissue sections were dewaxed and rehydrated. Antigen retrieval was performed by incubating the slides in 10 mmol/L sodium citrate buffer and microwaving the samples for 20 minutes. After blocking with 3% H2O2 and 10% normal goat serum (NGS), the slides were incubated with rabbit polyclonal antibodies against Snail (1:200; GTX125918; GeneTex, Inc., San Antonio, TX, USA) or GFP (1:500; GTX 113617; GeneTex) at 4°C overnight. The slides were then incubated with a biotin‐conjugated anti‐rabbit secondary antibody followed by polymer‐HRP reagent, using an ABC kit from Vector Laboratories (Burlingame, CA, USA). Peroxidase activity was visualized with a diaminobenzidine tetrahydroxychloride solution (Vector Laboratories). The sections were counterstained with hematoxylin.
2.6. Statistical analysis
All observations were confirmed by at least 3 independent experiments. The results are presented as the means ± standard deviations. We used two‐tailed, paired Student's t tests for all pairwise comparisons. Comparisons between multiple groups were performed using one‐way ANOVA followed by Dunnett's t test. In all comparisons, differences were considered statistically significant at P < .05.
More detailed information is provided in Data S1.
3. RESULTS
3.1. Overexpression of TIP30 inhibits TGF‐β‐induced EMT and invasion of lung cancer cells
The presence of TGF‐β in the microenvironment of lung tumors is suggested to be the critical factor for the initiation of metastasis.24 We first examined the role of TIP30 in the TGF‐β‐induced EMT and invasion of 2 NSCLC cell lines, namely, H358 and PC13, which had a relatively lower TIP30 expression than that of the other tested cell lines (Figure 1A). Treatment with TGF‐β induced a morphological change in H358 and PC13 cells to a mesenchymal phenotype (Figure 1B). The occurrence of TGF‐β‐induced EMT was confirmed by western blotting and quantitative PCR, which showed the downregulation of E‐cadherin and the upregulation of N‐cadherin and vimentin at both the protein and mRNA levels (Figure 1C,D). The overexpression of TIP30 diminished the TGF‐β‐induced downregulation of E‐cadherin and upregulation of N‐cadherin and vimentin, but TIP30 alone had little effect on EMT (Figure 1E). Similarly, the TGF‐β‐induced alterations in E‐cadherin, N‐cadherin and vimentin mRNA expression were reversed by TIP30 overexpression (Figure 1F). On the other hand, the forced expression of TIP30 significantly inhibited TGF‐β‐promoted cell invasion (Figure 1G). These data indicated that TIP30 negatively regulated the TGF‐β‐induced EMT and invasion of NSCLC cells.
Figure 1.
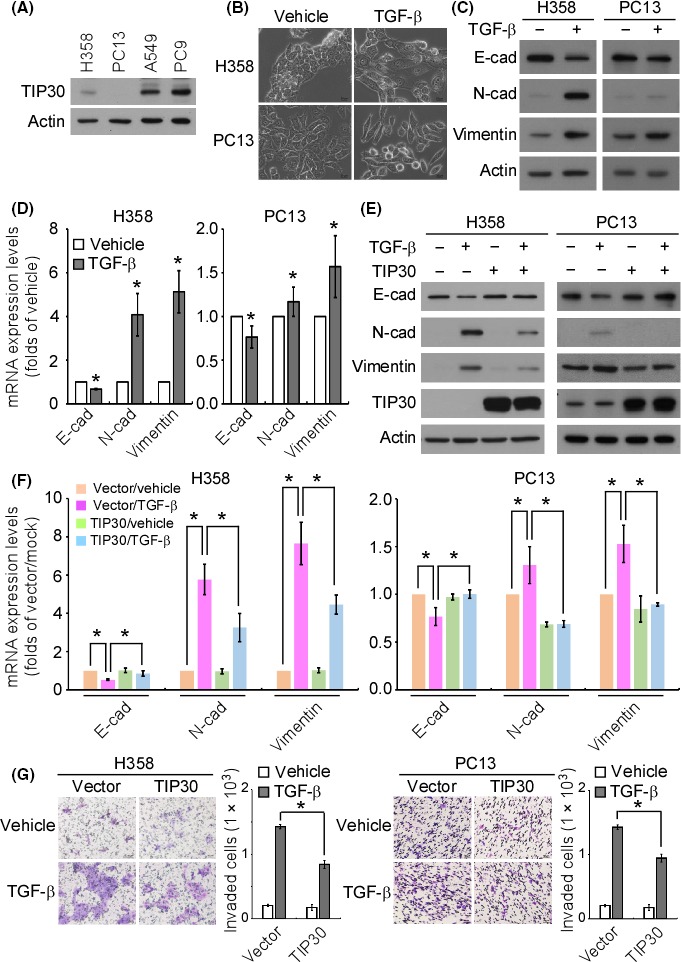
Overexpression of Tat‐interacting protein 30 (TIP30) suppressed tumor growth factor (TGF)‐β‐induced epithelial‐to‐mesenchymal transition (EMT) and invasion. (A) The endogenous TIP30 protein levels in the indicated cell lines were examined by western blotting. (B) Bright‐field images of H358 and PC13 cells treated with TGF‐β (10 ng/mL) for 72 h. Scale bar = 20 μm. (C) H358 and PC13 cells were treated with TGF‐β, and the expression of the indicated proteins was examined by western blotting. (D) The mRNA levels of the indicated genes in the TGF‐β‐treated H358 and PC13 cells were examined by quantitative PCR. *P < .05. (E) Stable clones of vector‐only and TIP30‐overexpressing H358 and PC13 cells were treated with TGF‐β. The expression of the indicated proteins was examined by western blotting. (F) The mRNA levels of the indicated genes in the TGF‐β‐treated stable clones of H358 and PC13 cells were examined by quantitative PCR. *P < .05. (G) The in vitro invasive ability of the TGF‐β‐treated stable clones of H358 and PC13 cells was examined by a transwell invasion assay. *P < .05
3.2. Knockdown of TIP30 induces EMT and promotes invasion and sphere formation
To study the role of TIP30 in EMT and cell invasion, TIP30 was stably knocked down in A549 and PC9 cells, which express high levels of TIP30, by introducing 2 clones of TIP30 shRNAs. The knockdown of TIP30 changed the cell morphology to an elongated and spindle‐like shape in both cell lines (Figure 2A). On the other hand, the knockdown of TIP30 downregulated E‐cadherin protein expression and upregulated the protein levels of N‐cadherin and vimentin compared to their levels in the scrambled control‐transfected cells (Figure 2B). Along with promoting EMT, the knockdown of TIP30 promoted the invasion of A549 and PC9 cells (Figure 2C). A previous study indicated that cells undergoing EMT develop stem cell properties.25 We therefore analyzed the mRNA expression of the stemness‐associated genes. The knockdown of TIP30 significantly upregulated the mRNA levels of Bmi1, CD133 and nestin compared to their levels in the scrambled control‐transfected cells. The mRNA levels of Nanog and Notch1 also showed a trend of upregulation in the TIP30 knockdown cells, although the difference was not statistically significant (Figure 2D). Consistently, the knockdown of TIP30 increased the number of tumor spheres, which represents the self‐renewal ability of the cells (Figure 2E).
Figure 2.
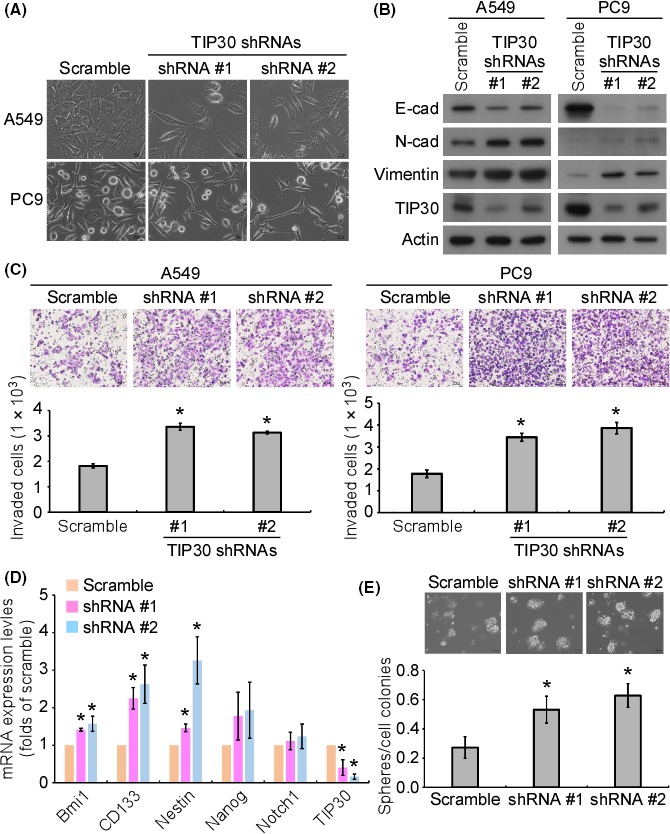
Knockdown of Tat‐interacting protein 30 (TIP30) promoted lung cancer cell epithelial‐to‐mesenchymal transition (EMT), invasion and stemness. (A) The bright‐field images of the scrambled and TIP30‐knockdown stable clones of A549 and PC9 cells. Scale bar = 20 μm. (B) The expression of the indicated proteins in the scrambled and TIP30 knockdown stable clones of A549 and PC9 cells was examined by western blotting. (C) The in vitro invasive ability of the scrambled and TIP30 knockdown stable clones of A549 and PC9 cells was examined by a transwell invasion assay. A quantitative analysis of the invading cells was performed. *P < .05. (D) The mRNA levels of the stemness‐related genes in the scrambled and TIP30 knockdown stable clones of A549 and PC9 cells were examined by quantitative PCR. *P < .05. € The sphere formation ability of the scrambled and TIP30‐knockdown stable clones of A549 and PC9 cells was examined. *P < .05
To further confirm the inhibitory function of TIP30 on EMT and invasion, TIP30 was knocked out in A549 cells by using the clustered regularly interspaced shor palindromiv repeats (CRISPR)‐CRISPR‐associated protein 9 (Cas9) genome editing system. The loss of TIP30 resulted in the induction of the downregulation of E‐cadherin and upregulation of N‐cadherin and vimentin (Figure S1A). Furthermore, the knockout of TIP30 increased the invasive ability of A549 cells (Figure S1B).
3.3. Recombinant HIV1 Tat protein promotes EMT and invasion
Since TIP30 is a HIV1 Tat‐interacting protein, we next studied the effect of HIV1 Tat on the EMT and invasion of NSCLC cells. A549 and H358 cells were treated with recombinant Tat (rTat), which contains a protein transduction domain that allows Tat to penetrate the cell membrane.26 After rTat treatment, the downregulation of E‐cadherin and upregulation of N‐cadherin were observed in A549 and H358 cells, while an increase in vimentin and Snail expression was seen only in A549 cells (Figure 3A). In addition, treatment with rTat significantly promoted the in vitro invasion of both A549 and H358 cells (Figure 3B). Cotreatment with TGF‐β and BSA elicited an apparent EMT effect after 1.5 days, but combined treatment with TGF‐β and rTat induced EMT as early as 0.5 days post‐treatment (Figure 3C,D). In addition, lower doses of TGF‐β were sufficient to induce EMT when rTat was present in the culture medium (Figure 3E,F) than when rTat was absent.
Figure 3.
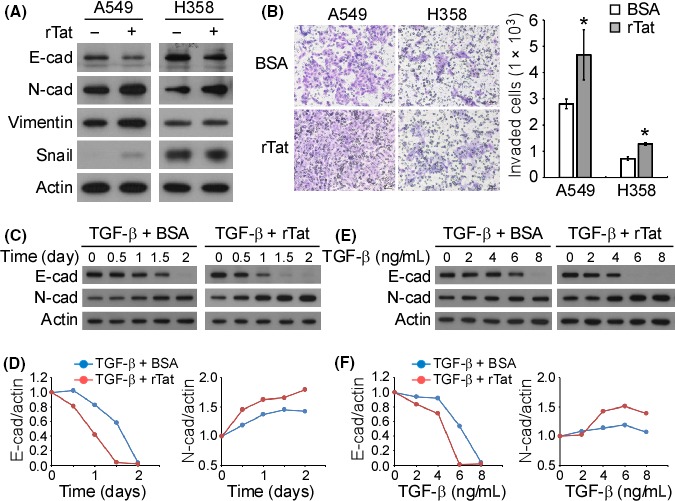
HIV1 Tat promotes epithelial‐to‐mesenchymal transition (EMT) and invasion. (A) A549 and H358 cells were treated with recombinant HIV1 Tat (rTat; 200 ng/mL) for 72 h, and the expression of the indicated proteins was examined by western blotting. (B) The in vitro invasive ability of rTat‐treated A549 and H358 cells was examined by a transwell invasion assay. A quantitative analysis of the invading cells was performed. *P < .05. (C) A549 cells were treated with tumor growth factor (TGF)‐β (10 ng/mL) or TGF‐β + rTat (50 ng/mL) for the indicated durations. Western blotting was performed to examine the expression of the indicated proteins. The chemiluminescence signals on the blot were quantified and are plotted in (D). (E) A549 cells were treated with different concentrations of TGF‐β or TGF‐β + rTat (50 ng/mL) for 72 h. Western blotting was performed to examine the expression of the indicated proteins. The chemiluminescence signals on the blot were quantified and are plotted in (F)
3.4. Binding of HIV1 Tat to TIP30 promotes the nuclear translocation of Snail
Previous studies demonstrated that TIP30 interacted with importin‐β and inhibited the nuclear translocation of many transcriptional regulators.23, 27, 28 Consistent with these findings, an in silico docking model showed that TIP30 directly bound to importin‐β through the α‐5 and α‐6 helices of importin‐β (Figure S2A). Interestingly, the docking model also predicted that TIP30 bound to HIV1 Tat by using the same domain that interacts with importin‐β (Figure S2B). Accordingly, we hypothesized that HIV1 Tat may block the interaction of TIP30 with importin‐β. An immunoprecipitation assay using a TIP30 antibody showed that TIP30 interacted with importin‐β (Figure 4A). However, the ectopically expressed HIV1 Tat‐Flag protein abolished the interaction between TIP30 and importin‐β (Figure 4A). We next asked whether the interaction of TIP30 with importin‐β may block the binding of Snail to importin‐β. Immunoprecipitation with an anti‐importin‐β antibody showed that the interaction between Snail and importin‐β was diminished by the forced expression of TIP30 (Figure 4B). These data suggested that TIP30 competes with Snail for binding to importin‐β. Indeed, the results of the western blot analysis showed that the Snail protein level was higher in the nuclear fraction of TIP30 knockdown cells than it was in the nuclear fraction of scrambled control cells (Figure 4C). The immunofluorescence analysis also showed an increased nuclear accumulation of Snail in TIP30‐knockdown cells (Figure 4D). On the other hand, the ectopic expression of HIV1 Tat‐Flag in A549 cells also promoted the nuclear translocation of Snail (Figure 4E). In contrast, the overexpression of TIP30 inhibited the nuclear translocation of Snail in H358 cells, which express relatively lower levels of endogenous TIP30 than do A549 cells, and the co‐expression of HIV1 Tat‐Flag and TIP30 reversed the inhibitory function of TIP30 on the nuclear translocation of Snail (Figure 4F). In addition, the knockout of TIP30 increased the nuclear translocation of Snail in TIP30 knockout A549 cells (Figure S1C,D). These results indicated that TIP30 negatively regulated the nuclear translocation of Snail. Moreover, HIV1 Tat blocked the TIP30‐importin‐β interaction, which increased the interaction of Snail with importin and promoted the nuclear translocation of Snail.
Figure 4.
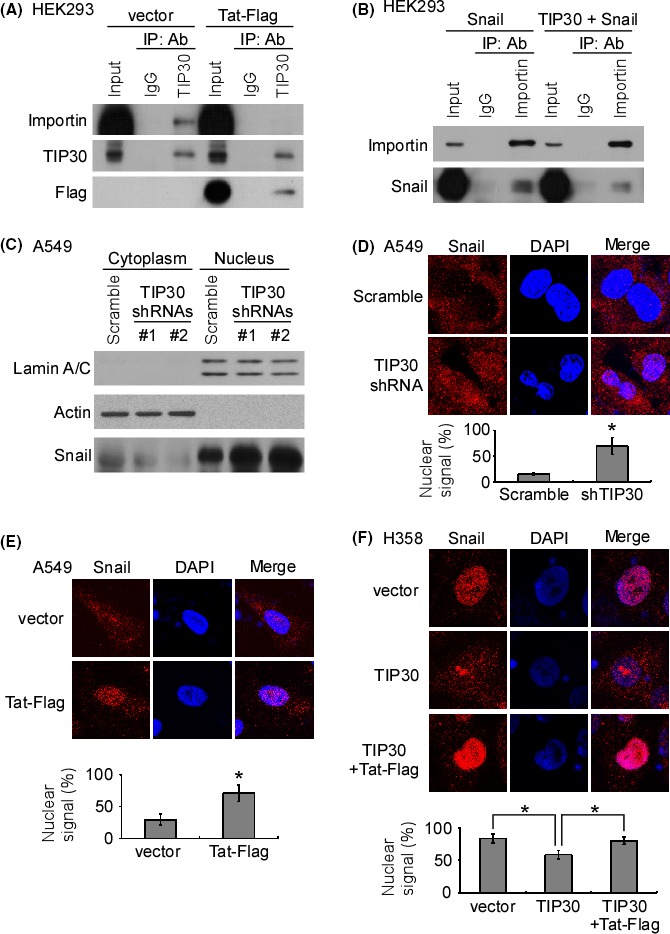
Interaction of HIV1 Tat and Tat‐interacting protein 30 (TIP30) promotes the nuclear translocation of Snail. (A) Human embryonic kidney 293 (HEK293) cells were transfected with a combination of either vector/TIP30/importin‐β plasmids or HIV1 Tat‐Flag/TIP30/importin‐β plasmids. Immunoprecipitation was performed using anti‐IgG or anti‐TIP30 antibodies. The levels of the indicated proteins were examined by western blotting. (B) HEK293 cells were transfected with a combination of either vector/Snail/importin‐β plasmids or TIP30/Snail/importin‐β plasmids. Immunoprecipitation was performed using anti‐IgG or anti‐importin‐β antibodies. The levels of the indicated proteins were examined by western blotting. (C) Western blotting was performed to examine the expression of the indicated proteins in the cytoplasmic and nuclear fractions of the scrambled and TIP30 knockdown stable clones of A549 cells. (D) Representative confocal images of the subcellular localization of Snail in the scrambled and TIP30 knockdown stable clones of A549 cells. (E) Representative confocal images of the subcellular localization of Snail in the vector and Tat‐Flag‐overexpressed stable clones of A549 cells. (F) Representative confocal images of the subcellular localization of Snail in the vector and TIP30, and a combination of TIP30 and Tat‐Flag‐overexpressed stable clones of H358 cells. *P < .05
3.5. HIV1 Tat reverses the TIP30‐induced inhibition of lung cancer metastasis
To examine whether TIP30 inhibits lung cancer metastasis and to assess the role of HIV1 Tat in TIP30‐regulated metastasis, a nude mouse model of GFP‐ and luciferase‐labeled H358 cell (H358‐GL) transplantation was used. Vector‐only (vector), TIP30‐overexpressing (TIP30), or TIP30 and Tat‐Flag‐co‐expressing (TIP30 + Tat‐Flag) H358‐GL cells were orthotopically transplanted into the left lung of nude mice. The metastasis of the cancer cells was monitored by detecting the bioluminescence signal in the right lung of the individual mice. Mice that received the H358‐GL‐TIP30 cell transplantation had the lowest bioluminescence intensity in the right lung, compared to the mice that received the H358‐GL‐vector or H358‐GL‐TIP30 + Tat‐Flag cell transplantation (Figure S3). Fifty days post‐transplantation, the lungs were removed and examined for metastases. The primary tumors in the left lung were clearly seen in all the mice in the three experimental groups (Figure 5A). The mice that received the H358‐GL‐vector cell transplantation showed noticeable metastatic nodules and bioluminescence signals in the right lung. The number of tumor nodules in the right lung of the H358‐GL‐TIP30 cell‐transplanted mice was greatly reduced compared with that in the right lungs of the H358‐GL‐vector cell‐transplanted mice, suggesting an inhibitory effect of TIP30 on tumor metastasis. The co‐expression of TIP30 and HIV1 Tat‐Flag significantly increased the number of metastatic nodules in the right lung of the mice (Figure 5A). The histological analysis and IHC using a GFP antibody confirmed that the primary and metastatic tumors originated from the transplanted cells (Figure 5B). A representative IHC image shows that the Snail protein was mainly localized in the nuclei of the transplanted H358‐GL‐vector and H358‐GL‐TIP30 + Tat‐Flag cells, whereas the cytoplasmic localization of Snail was observed in the H358‐GL‐TIP30 cells (Figure 5C). The statistical analysis confirmed that the nuclear translocation of Snail was inhibited by TIP30 overexpression and that the co‐expression of HIV1 Tat‐Flag and TIP30 promoted the nuclear localization of Snail (Figure 5C). Similar results were observed by using immunofluorescent labelling of Snail in the transplanted tumor cells (Figure S4). Our in vitro study showed that HIV1 Tat inhibited that inhibitory role of TIP30 on EMT. The IHC results showed that the ectopic expression of TIP30 increased the expression levels of E‐cadherin in the H358‐TIP30 xenograft tumors compared to the levels of E‐cadherin in the H358‐vector tumors (Figure 5D). On the other hand, co‐expression of TIP30 and Tat‐Flag reduced the E‐cadherin expression compared to the TIP30‐overexpressed tumors. These data confirmed that TIP30 played an inhibitory role on EMT, and the HIV1 Tat promoted EMT and invasion through inhibiting the functions of TIP30.
Figure 5.
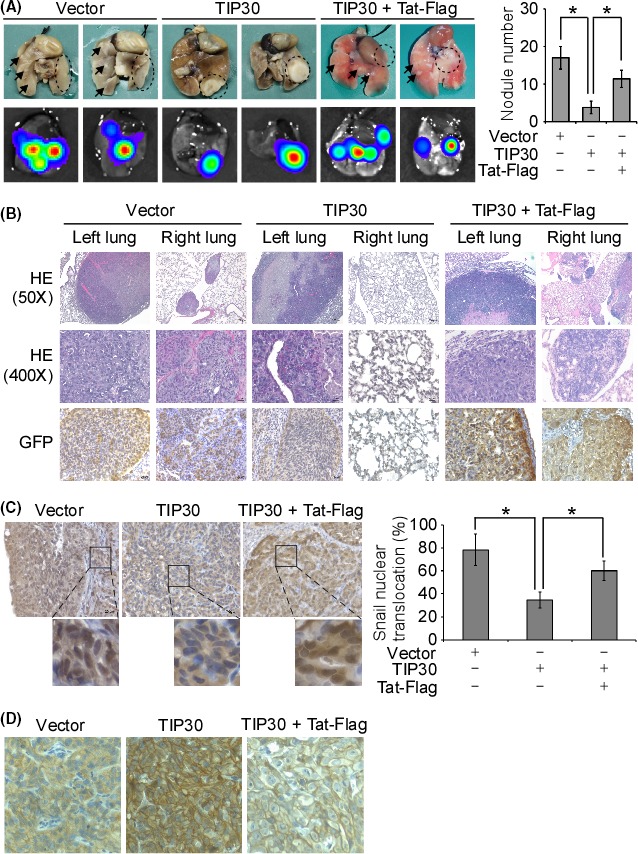
HIV1 Tat diminished the inhibitory effect of Tat‐interacting protein 30 (TIP30) overexpression on metastasis in an orthotopic mouse model. (A) At the end of the animal experiments, the lungs were removed, and IVIS images of the lungs were captured. The number of visible nodules was counted. *P < .05. The arrows indicate the nodules in the right lungs. The dashed open circles indicate the primary tumors in the left lungs. (B) Representative images show the hematoxylin and eosin staining and immunohistochemical analysis of GFP in the tissue sections from the primary and metastatic tumors in the left and right lungs, respectively. The magnification is 50× or 400×, as indicated. (C) The representative images show the results of immunohistochemistry (IHC) for Snail in the tissue sections from each experimental group. The percentage of cells with a signal for Snail staining in the nucleus was calculated. *P < .05. (D) The representative images show the results of IHC for E‐cadherin expression in the tumor tissues of each experimental group
4. DISCUSSION
Although lung cancer cells are not the major targets of HIV infection, the Tat protein released from HIV‐infected immune cells may affect neighboring epithelial cells in the tumor mass. For example, a Tat‐containing supernatant from an HIV‐infected H9 human embryonic cell line promotes the development and progression of Kaposi's sarcoma cells.29 In fact, the Tat concentration in the plasma of HIV‐infected patients is low, between 1 and 40 ng/mL.30, 31 However, one can suspect that the infiltration of HIV‐infected CD4 T cells into tumors may elevate the Tat concentration in the local microenvironment.32 To mimic the pathological condition, the concentrations of recombinant HIV1 Tat used in this study were between 50 and 200 ng/mL for the Western blot analysis of the TGF‐β‐induced EMT and invasion of NSCLC cells. The use of these concentrations is consistent with previous studies showing that Tat at a concentration of 200 ng/mL promotes brain pericyte migration,33 induces the expression of multidrug‐resistant protein 1 in brain microvascular endothelial cells,34 and suppresses Toll‐like receptor 4 expression in cholangiocytes.35
In addition to TIP30, other Tat‐interacting proteins have been identified. HIV1 Tat‐interacting protein of 110 kDa (TIP110) functions as a nuclear RNA‐binding protein and controls proliferation, survival, and differentiation; in addition, it is a potential cancer antigen for immunotherapy.36 The HIV1 Tat‐interacting protein of 60 kDa (TIP60) is a histone acetyltransferase that is linked to the DNA damage response and to cell migration, stemness maintenance and chemoresistance.37 Although the roles of HIV Tat‐interacting proteins in many different cancers have been discovered, the effect of the Tat protein itself in lung cancer development and progression is completely unknown. In our study, the application of the recombinant Tat protein alone was sufficient to induce EMT and to promote the invasion of A549 and H358 cells without TGF‐β treatment. Although Snail expression levels were undetectable in the control group of A549 cells, Snail protein expression was upregulated after treatment with the recombinant Tat protein. Our data indicated that the binding of Tat to TIP30 contributed to the nuclear translocation of Snail, leading to EMT and an increased invasive ability. However, the mechanism of the upregulation of Snail as the initial response to Tat treatment is unclear. Further experiments are required to study whether other Tat‐interacting proteins that are involved in Snail expression may be affected by Tat treatment.38
Within the N‐terminal region of TIP30, there is an adenosine triphosphate‐binding motif driving TIP30 kinase activity, which is required for the phosphorylation of RNA polymerase II and the enhancement of Tat‐activated transcription.15 Additionally, TIP30 contains a highly conserved β‐α‐β motif, which binds to NADPH and drives TIP30 reductase activity.39 In addition to its enzymatic role, the binding of NADPH to TIP30 is responsible for the interaction of TIP30 and importin‐β.28 Consistent with a previous finding in HCC, our study showed that the interaction of HIV1 Tat and TIP30 inhibited the binding of TIP30 to importin‐β, thus leading to a decrease in the nuclear translocation of Snail. It is unclear whether HIV1 Tat binds directly to TIP30 or interacts with TIP30 through the formation of a protein complex,13 but the interaction of HIV1 Tat with TIP30 may dissociate the TIP30‐RanGTP‐importin‐β protein complex. In contrast, Snail may directly bind to importin through the Snail zinc finger domain. The Snail‐RanGTP‐importin‐β complex may reform and contribute to the nuclear localization of Snail after the dissociation of TIP30 and importin‐β. However, the detailed mechanism of the protein‐protein interaction between HIV1 Tat, TIP30, Snail and importin‐β should be extensively investigated in the future.
In the general population without HIV infection, the loss of TIP30 is frequently found in tumors of late‐stage NSCLC patients.5 The decreased TIP30 expression in metastatic tumors might be regulated by epigenetic mechanisms, including miRNAs and the hypermethylation of the TIP30 promoter.22, 40 It is unknown whether this regulation of TIP30 also occurs in lung cancer patients with HIV infection. Unfortunately, most lung cancer patients with HIV do not receive surgery as their treatment. Future study using clinical samples from NSCLC patients with HIV infection may contribute to the understanding of the correlation between Tat‐TIP30 interaction and disease progression. Our study demonstrated that the exposure of lung cancer cells to extracellular HIV1 Tat, which leads to the inhibition of TIP30 function, could be one of the mechanisms underlying the poorer prognosis and increased metastasis observed in lung cancer patients with HIV infection.
In summary, we have presented herein the novel finding that the downregulation of TIP30 or the presence of HIV1 Tat contributes to NSCLC cell EMT, invasion and metastasis. TIP30 competes with Snail for binding to importin‐β and inhibits the nuclear translocation of Snail, which promotes cancer cell EMT and invasion. However, the binding of HIV1 Tat to TIP30 blocks the interaction between TIP30 and importin‐β, leading to an increase in the nuclear translocation of Snail and to the invasion and distant metastasis of NSCLC cells (Figure 6). Taken together, these results suggest that the penetration of the HIV1 Tat protein may play an important role in tumor progression in NSCLC by enhancing cell mobility and invasion and, consequently, promoting the distant metastasis of NSCLC cells by regulating the intracellular distribution of Snail.
Figure 6.
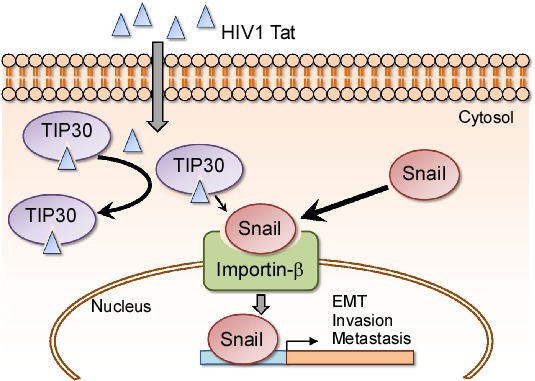
Schematic of the regulatory mechanism of the nuclear translocation of Snail via HIV1 Tat‐TIP30 (Tat‐interacting protein 30) interaction. TIP30 competes with Snail for binding to importin‐β and inhibits the nuclear translocation of Snail. In addition, the binding of HIV1 Tat to TIP30 blocks the interaction between TIP30 and importin‐β, leading to both the increase in Snail nuclear translocation and the invasion and distant metastasis of non‐small cell lung cancer (NSCLC) cells
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This study is financially supported by the Ministry of Science and Technology of Taiwan (MOST 107‐2320‐B‐037‐025‐ and MOST 106‐2320‐B‐037‐022‐). This study is supported partially by Kaohsiung Medical University “Aim for the Top Universities Grant, grant No. KMU‐TP103E04, KMU‐TP104E05, and KMU‐TP105E05”. This work was also financially supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. Guide RNAs were obtained from the National RNAi Core Facility Platform located at the Institute of Molecular Biology/Genomic Research Center, Academia Sinica, supported by the National Core Facility Program for Biotechnology Grants of NSC (NSC 100‐2319‐B‐001‐002). We thank the Center for Research Resources and Development at Kaohsiung Medical University for the instrumental support of a confocal microscope. We acknowledge the Laboratory Animal Facility of Kaohsiung Medical University for providing the IVIS imaging system and technical supports.
Liu Y‐P, Chen C‐H, Yen C‐H, et al. Human immunodeficiency virus Tat‐TIP30 interaction promotes metastasis by enhancing the nuclear translocation of Snail in lung cancer cell lines. Cancer Sci. 2018;109:3105–3114. 10.1111/cas.13768
REFERENCES
- 1. Yoshimura K. Current status of HIV/AIDS in the ART era. J Infect Chemother. 2017;23(1):12‐16. [DOI] [PubMed] [Google Scholar]
- 2. Pierre S, Riviera V, Jean CP, et al. Live with the disease like you used to before you knew you were infected: a qualitative study among 10‐year survivors living with HIV in Haiti. AIDS Patient Care STDS. 2017;31(3):145‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chiu CG, Smith D, Salters KA, et al. Overview of cancer incidence and mortality among people living with HIV/AIDS in British Columbia, Canada: implications for HAART use and NADM development. BMC Cancer. 2017;17(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suneja G, Shiels MS, Angulo R, et al. Cancer treatment disparities in HIV‐infected individuals in the United States. J Clin Oncol. 2014;32(22):2344‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sigel K, Crothers K, Dubrow R, et al. Prognosis in HIV‐infected patients with non‐small cell lung cancer. Br J Cancer. 2013;109(7):1974‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fiume G, Scialdone A, Albano F, et al. Impairment of T cell development and acute inflammatory response in HIV‐1 Tat transgenic mice. Sci Rep. 2015;5:13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lorenzo‐Redondo R, Fryer HR, Bedford T, et al. Persistent HIV‐1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahimian P, He JJ. Exosome‐associated release, uptake, and neurotoxicity of HIV‐1 Tat protein. J Neurovirol. 2016;22(6):774‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao S, Hu M, Hao T, et al. MiRNA‐891a‐5p mediates HIV‐1 Tat and KSHV Orf‐K1 synergistic induction of angiogenesis by activating NF‐kappaB signaling. Nucleic Acids Res. 2015;43(19):9362‐9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fields JA, Dumaop W, Crews L, et al. Mechanisms of HIV‐1 Tat neurotoxicity via CDK5 translocation and hyper‐activation: role in HIV‐associated neurocognitive disorders. Curr HIV Res. 2015;13(1):43‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bukrinsky M. SNFing HIV transcription. Retrovirology. 2006;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jean MJ, Power D, Kong W, Huang H, Santoso N, Zhu J. Identification of HIV‐1 Tat‐associated proteins contributing to HIV‐1 transcription and latency. Viruses. 2017;9(4). 10.3390/v9040067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xiao H, Tao Y, Greenblatt J, Roeder RG. A cofactor, TIP30, specifically enhances HIV‐1 Tat‐activated transcription. Proc Natl Acad Sci USA. 1998;95(5):2146‐2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker ME. TIP30, a cofactor for HIV‐1 Tat‐activated transcription, is homologous to short‐chain dehydrogenases/reductases. Curr Biol. 1999;9(13):R471. [DOI] [PubMed] [Google Scholar]
- 15. Xiao H, Palhan V, Yang Y, Roeder RG. TIP30 has an intrinsic kinase activity required for up‐regulation of a subset of apoptotic genes. EMBO J. 2000;19(5):956‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu X, Li Z, Wu WK. TIP30: a novel tumor‐suppressor gene. Oncol Res. 2014;22(5–6):339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Cao X, Dong W, Luo S, Suo Z, Jin Y. Expression of TIP30 tumor suppressor gene is down‐regulated in human colorectal carcinoma. Dig Dis Sci. 2010;55(8):2219‐2226. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Yang Z, Jiang S, et al. MCM2 and TIP30 are prognostic markers in squamous cell/adenosquamous carcinoma and adenocarcinoma of the gallbladder. Mol Med Rep. 2016;14(5):4581‐4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong X, Li K, Luo Z, et al. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am J Pathol. 2009;174(5):1931‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yin F, Sharen G, Yuan F, et al. TIP30 regulates lipid metabolism in hepatocellular carcinoma by regulating SREBP1 through the Akt/mTOR signaling pathway. Oncogenesis. 2017;6(6):e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu M, Yin F, Yang L, et al. Contribution of TIP30 to chemoresistance in laryngeal carcinoma. Cell Death Dis. 2014;5:e1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouyang H, Gore J, Deitz S, Korc M. microRNA‐10b enhances pancreatic cancer cell invasion by suppressing TIP30 expression and promoting EGF and TGF‐beta actions. Oncogene. 2014;33(38):4664‐4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu M, Yin F, Fan X, et al. Decreased TIP30 promotes Snail‐mediated epithelial‐mesenchymal transition and tumor‐initiating properties in hepatocellular carcinoma. Oncogene. 2015;34(11):1420‐1431. [DOI] [PubMed] [Google Scholar]
- 24. Borczuk AC, Sole M, Lu P, et al. Progression of human bronchioloalveolar carcinoma to invasive adenocarcinoma is modeled in a transgenic mouse model of K‐ras‐induced lung cancer by loss of the TGF‐beta type II receptor. Cancer Res. 2011;71(21):6665‐6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu YC, Tang SJ, Sun GH, Sun KH. CXCR7 mediates TGFbeta1‐promoted EMT and tumor‐initiating features in lung cancer. Oncogene. 2016;35(16):2123‐2132. [DOI] [PubMed] [Google Scholar]
- 26. Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10(7):290‐295. [DOI] [PubMed] [Google Scholar]
- 27. King FW, Shtivelman E. Inhibition of nuclear import by the proapoptotic protein CC3. Mol Cell Biol. 2004;24(16):7091‐7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El Omari K, Bird LE, Nichols CE, Ren J, Stammers DK. Crystal structure of CC3 (TIP30): implications for its role as a tumor suppressor. J Biol Chem. 2005;280(18):18229‐18236. [DOI] [PubMed] [Google Scholar]
- 29. Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong‐Staal F. Tat protein of HIV‐1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345(6270):84‐86. [DOI] [PubMed] [Google Scholar]
- 30. Westendorp MO, Frank R, Ochsenbauer C, et al. Sensitization of T cells to CD95‐mediated apoptosis by HIV‐1 Tat and gp120. Nature. 1995;375(6531):497‐500. [DOI] [PubMed] [Google Scholar]
- 31. Xiao H, Neuveut C, Tiffany HL, et al. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV‐1. Proc Natl Acad Sci USA. 2000;97(21):11466‐11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor JG, Liapis K, Gribben JG. The role of the tumor microenvironment in HIV‐associated lymphomas. Biomark Med. 2015;9(5):473‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niu F, Yao H, Zhang W, Sutliff RL, Buch S. Tat 101‐mediated enhancement of brain pericyte migration involves platelet‐derived growth factor subunit B homodimer: implications for human immunodeficiency virus‐associated neurocognitive disorders. J Neurosci. 2014;34(35):11812‐11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayashi K, Pu H, Andras IE, et al. HIV‐TAT protein upregulates expression of multidrug resistance protein 1 in the blood‐brain barrier. J Cereb Blood Flow Metab. 2006;26(8):1052‐1065. [DOI] [PubMed] [Google Scholar]
- 35. O'Hara SP, Small AJ, Gajdos GB, Badley AD, Chen XM, Larusso NF. HIV‐1 Tat protein suppresses cholangiocyte toll‐like receptor 4 expression and defense against Cryptosporidium parvum. J Infect Dis. 2009;199(8):1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whitmill A, Liu Y, Timani KA, Niu Y, He JJ. Tip110 deletion impaired embryonic and stem cell development involving downregulation of stem cell factors Nanog, Oct4, and Sox2. Stem Cells. 2017;35(7):1674‐1686. [DOI] [PubMed] [Google Scholar]
- 37. Banerjee Mustafi S, Chakraborty PK, Naz S, et al. MDR1 mediated chemoresistance: BMI1 and TIP60 in action. Biochim Biophys Acta. 2016;1859(8):983‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Subbaiah VK, Rajagopalan D, et al. TIP60 inhibits metastasis by ablating DNMT1‐SNAIL2‐driven epithelial‐mesenchymal transition program. J Mol Cell Biol. 2016;8(5):384‐399. [DOI] [PubMed] [Google Scholar]
- 39. Baker ME, Yan L, Pear MR. Three‐dimensional model of human TIP30, a coactivator for HIV‐1 Tat‐activated transcription, and CC3, a protein associated with metastasis suppression. Cell Mol Life Sci. 2000;57(5):851‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dong W, Shen R, Cheng S. Reduction of TIP30 in esophageal squamous cell carcinoma cells involves promoter methylation and microRNA‐10b. Biochem Biophys Res Commun. 2014;453(4):772‐777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


