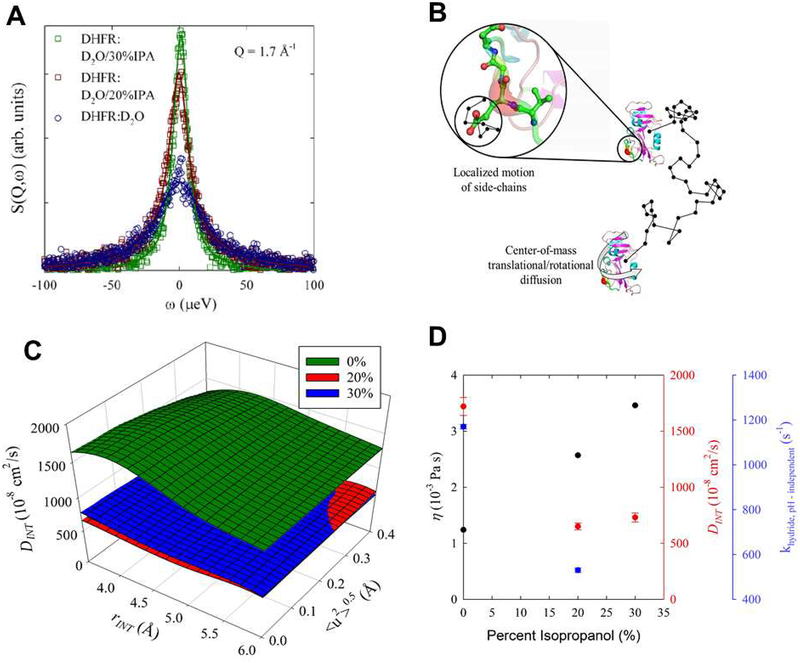
Figure 5: Effect of IPA on DHFR dynamics.
(A) Background subtracted quasi-elastic neutron scattering (QENS) data from DHFR hydrogen atoms in solution at the indicated IPA concentration. The points show experimental data and line indicates fit to the model as described in supporting information. (B) The contribution to the QENS signal comes from hydrogen local motions and the translational and center-of-mass rotational movement of the entire enzyme in solution. A random path is illustrated by the black line where the center-of-mass translational and rotational motion of DHFR will be affected by the viscosity of the solution. The local dynamics of hydrogens (DINT) attached to the side chain were modeled as diffusion in a sphere. (C) The internal motion diffusion coefficient, DINT, at the indicated IPA solvent concentrations. The plot shows the sensitivity of DINT at fixed values of the radius defining the extent of internal motion and the vibrational, oscillation Debye-Waller factor. As IPA concentration is initially increased to 2 0 %, DINT decreases by approximately 60%. There is little difference between the 2 0 % and 30% samples. (D) Dependence of viscosity (η), DINT, and pH independent rate for hydride transfer (khydride,pH ‒ independent) with isopropanol concentration. The first two quantities are for deuterated solutions while the last is from protonated solutions. Note, the uncertainties associated with the viscosity measurements are less than the size of the symbols. DINT is from Table S3.