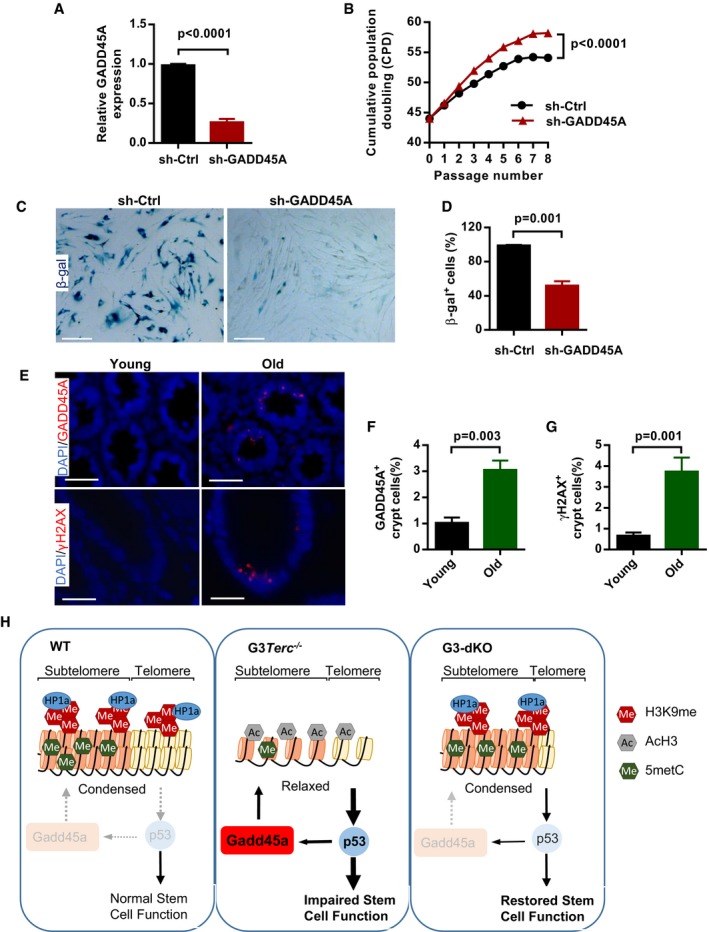
-
A
The knockdown efficiency of shRNA‐GADD45A in human WI38 fibroblasts (n = 3 replicates per group).
-
B
The proliferation curves of human WI38 fibroblasts treated with control shRNA or shRNA against GADD45A (n = 3 replicates per group).
-
C, D
Representative images of β‐gal staining in human fibroblast cultures with control and shRNA‐GADD45A treatments at passage 54 (C). Quantification of the percentage of β‐gal‐positive cells in human fibroblasts with control and shRNA‐GADD45A treatments (duplicates per group) (D).
-
E–G
Representative images of GADD45A and γH2AX staining in colon sections from young (35–50 y/o) and old (75–85 y/o) people (E). Quantification of the percentage of GADD45A‐positive cells in young and old human colon tissues (n = 8 per age group) (F). Quantification of the percentage of γH2AX‐positive cells in young and old human colon tissues (n = 6 per age group) (G).
-
H
Model for the role of Gadd45a in regulation of DNA damage response initiation. In G3Terc
−/− mice, Gadd45a promotes the accessibility of telomeric heterochromatin and enhances the DNA damage response initiation (e.g., pATM activation) through a BER‐dependent pathway. The initiated DNA damage response further causes upregulation of p53 and p21, which leads to impairment of intestine stem cell maintenance and function. In G3‐dKO mice, the telomeric heterochromatin is condensed and damage response initiation is reduced upon Gadd45a deletion, which further leads to decreased expression of p53 and p21. Therefore, the maintenance and function of intestine stem cell are improved.
Data information: Scale bars represent 150 μm in (C); 20 μm and 10 μm in (E) upper and lower panel. Data are presented as mean ± SEM.
P‐values were calculated with unpaired two‐tailed Student's
t‐test.