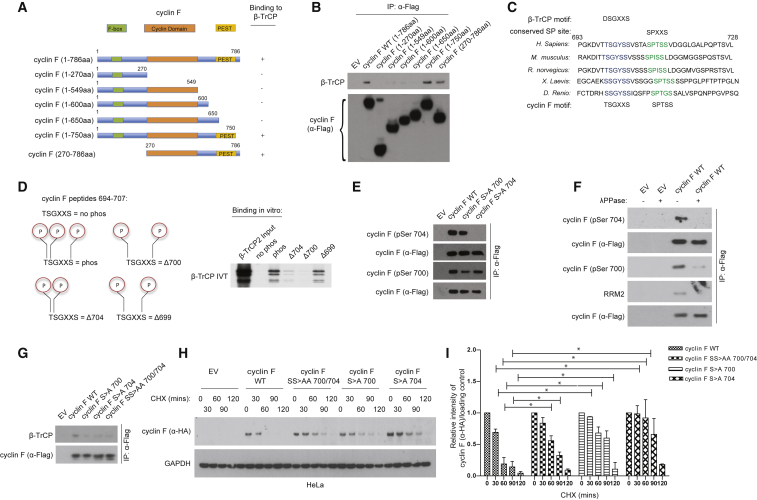
Figure 2.
A Non-canonical DSGXXS Motif Is Necessary for β-TrCP1 and β-TrCP2 Binding to Cyclin F
(A) Schematic representation of cyclin F WT and truncated fragments, highlighting F-box, the cyclin domain, and PEST. Cyclin F fragments interacting with endogenous β-TrCP are designated with the + symbol.
(B) HEK293T cells transfected with an empty vector (EV), FLAG-tagged cyclin F WT, or FLAG-tagged cyclin F truncated fragments were immunoprecipitated (IP) and immunoblotted as indicated.
(C) Alignment of cyclin F orthologs highlighting the putative β-TrCP binding motif and a conserved serine proline (SP) site.
(D) In vitro-transcribed and -translated β-TrCP2 labeled with methionine 35S was incubated at 30°C with beads coupled to the following peptides: 694-GKDVTTSGYSSVST-707 (no-phos), 694-GKDVTTpSpGYSSpVST-707 (phos), 694-GKDVTTSpGYSSpVST-707 (Δ699), 694-GKDVTTpSGYSSpVST-707 (Δ700), and 694-GKDVTTpSpGYSSVST-707 (Δ704). After 30 min, beads were washed, and bound β-TrCP2 was detected by autoradiography.
(E) HEK293T cells transfected with indicated plasmids were IP with anti-FLAG and immunoblotted as indicated.
(F) HeLa cells stably expressing an empty vector (EV) and FLAG-tagged cyclin F WT were IP. Immunoprecipitates were dephosphorylated by treatment with lambda phosphatase (λPPase±) and immunoblotted as indicated.
(G) HeLa cells stably expressing cyclin F WT and S > A 700, S > A 704, and SS > AA 700/704 mutants were IP and immunoblotted as indicated.
(H) HeLa cells stably expressing cyclin F WT and S > A 700, S > A 704, and SS > AA 700/704 were treated with cycloheximide (CHX) in minutes and immunoblotted as indicated.
(I) Densitometry analysis of cyclin F WT and S > A 700, S > A 704, and SS > AA 700/704 half-life. Results are means ± SEM of three independent experiments. Student’s t test. ∗p < 0.05.