Abstract
Metastases from renal clear cell carcinoma (RCCC) to the head and neck (HN) region are rare, representing 8–14% of all RCCC metastases, with the thyroid gland being the most common site of RCCC metastasis in the HN. Metastatic tumors that are located in the salivary glands have a prevalence of 5%, while the submandibular gland is only involved in 1% of the cases. We present the case of a 74-year-old female patient with metastasis to the submandibular gland, 11 years after radical nephrectomy for a RCCC.
INTRODUCTION
Renal clear cell carcinoma (RCCC) represents 2–3% of all malignant tumors and 85% of the kidney’s primary malignancies [1]. The different sites where the RCCC can produce metastasis are related to the kidney’s great vascular flow and the tumor capacity to adapt to very different vascular microenvironments [1–3]. The most frequent sites of metastasis are the lungs, liver, brain, skin [2–4], adrenal glands, bones, contralateral kidney and retroperitoneum [1]. Metastases are present, at the moment of diagnosis, in 25% of the cases, and have developed in 50% of the patients by later follow up [4].
The frequency of RCCC's metastases to the head and neck (HN) region vary between 8 and 15% according to the different series reported [1–6]. Although the thyroid gland represents the most frequent site of metastasis, the skin, nasal cavity, hard palate, paranasal sinus, tonsils, lips and gums are not uncommon localizations. The parotid gland is the most commonly compromised gland, whereas it is extremely rare to find metastasis in the submandibular gland. The secondary neoplasms of the salivary glands represent 5% of all salivary glands tumors, mainly from skin tumors of the HN region (85%). [5]
We present the case of a 74-year-old patient with metastasis to the submandibular gland, 11 years after radical nephrectomy for RCCC.
CASE REPORT
A 74-year-old woman, with a history of RCCC and a right radical nephrectomy 11 years before, consulted with a 4-month evolution hard nodule, of ~1.5 cm on the right submandibular gland. The ultrasound (US) examination showed a 16 mm hypoechogenic nodule with central vascularization. A fine needle aspiration biopsy was performed, demonstrating nests of epithelial cells with clear cytoplasm and rounded nuclei with anisocaryosis and presence of nucleoli. Additionally, immunohistochemistry (IHC) exams were requested and the biopsy was found positive for paired-box gene 8 (PAX 8). The histological characteristics and the IHC findings associated the nodule as being renal in origin.
The staging exams (Fig. 1) revealed multiple solid heterogeneous isointense lesions on the left kidney, consistent with multicentric RCCC. Greater omentum and left suprarenal gland nodules were also described. With the previous findings, the patient underwent systemic treatment with pazopanib (a tyrosine kinase inhibitor) for 12 months, showing a poor oncologic response. A surgical approach was decided.
Figure 1:
The white arrow shows the right submandibular gland in (A) magnetic resonance in T1 and (B) CT scan.
A partial left nephrectomy associated with adrenalectomy, enucleation of the nodules in the greater omentum and a right submaxillary gland removal was performed. The patient evolved with no postoperative complications.
The histopathological analysis of the right submandibular gland informed: tumor proliferation composing atypical epithelial cells with pleomorphic, hyperchromatic nuclei with prominent nucleoli and clear cytoplasm. They were arranged forming nests and acini surrounded by a delicate fibrovascular network, with areas of hemorrhage with deposits of hemosiderin and stroma with hyaline degeneration. The lesion invaded the submandibular gland (Fig. 2). The results confirmed an RCCC origin.
Figure 2:
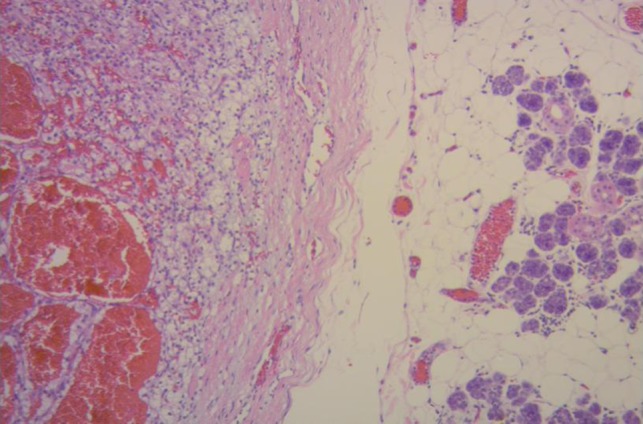
Renal clear cell carcinoma metastasis to submandibular gland (right). Submandibular glandular parenchyma with a well circumscribed tumor (left) composed of an alveolar, tubular and cystic, clear cell tumor with distinct but delicate cell boundaries and a characteristic network of small, thin walled vasculature. (Hematoxylin–eosin ×100.)
DISCUSSION
RCCC is the most common primary renal tumor. It is distinguished by its remarkable metastatic potential, related to the fact that: (i) it receives 25% of the effective circulating volume and (ii) it has a great capacity to adapt to different vascular microenvironments [3, 7]. The HN metastases of the RCCC represent ~16% of the cases [5], however, metastasis to the salivary glands are very rare.
The primary tumors of the salivary glands represent 3% of the neoplasms that affect the HN region, and when studying a mass, they should be the first diagnostic assumption. However, <1% of the salivary gland tumors are metastases and the most common primary tumors behind these metastases are squamous cell carcinomas of the HN [2]. The parotid gland is the salivary gland most frequently affected by metastasis (85%), while the submandibular gland is affected in only 15% of the cases.
Regarding metastases of RCCC to the submandibular gland, there are only seven cases reported in the literature [1–6], with one being located on the Wharton duct [6]. Although infrequent, metastases from RCCC must have a differential diagnosis in patients presenting with a submandibular mass. Moreover, a submandibular mass could be the first clinical symptom of an occult carcinoma [1, 5].
All the cases reported were with elderly adults, with the mean age at the moment of diagnosis being 70.2 years old (r60–97). The time interval between diagnosis and the development of metastasis was variable, from months up to more than 10 years later [3]. The critical period in which the metastasis appears is from 3 to 5 years after diagnosis, nevertheless, as many as 10% of the patients develop metastasis after 5 years [4]. From the eight cases reported, four had previous renal surgery and the mean time from surgery to the diagnosis of metastasis was 9 years and 4 months (r7–11). It is remarkable that all the metastases to the submandibular gland appeared at least 7 years after the renal surgery. Our case has the longest period of time between surgery and submandibular metastasis: 11 years after surgery.
The motive of the consultation in all cases was a painless mass, except for one case who consulted with a painful mass [1]. Regarding the localization of the metastasis, 6/8 were in the right submandibular gland, 1 in the left gland and there is no data about the other case. The relation between sides was homolateral in two cases (including our case), contralateral in one case and in the other cases it was not reported. Up to half of the cases had metastasis in other sites at the moment of diagnosis, with our case being one of them. The characteristics of the different series reported are presented in Table 1.
Table 1.
General characteristics of the reported series
| Author | N | Sex/Age | Time since renal surgery (years) | Localization | Presenting symptoms | Tumor size (mm) | Treatment | Other metastasis |
|---|---|---|---|---|---|---|---|---|
| Majewska et al. [5] | 1 | F/97 | NR | Right | Hard, cohesive, immobile mass | NR | NR | No |
| Balaban et al. [3] | 1 | F/66 | NA | Right | Mass | 13 × 13 | CMT–RDT | Yes (homolateral parotid gland, liver, lungs and brain) |
| Serouya et al. [4] | 1 | M/60 | 9 | Right (NR) | Mass | 12 | Submaxilectomy | No |
| Miah et al. [1] | 1 | F/61 | 7 | Right (contralateral) | Painful mass | 29 × 26 × 30 | Submaxilectomy | Yes (thyroid gland) |
| Moudouni et al. [2] | 1 | M/83 | 10 | Right (homolateral) | Mass | 40 × 30 | Submaxilectomy | No |
| Smits et al. (1984) | 1 | F/60 | NR | NR | NR | NR | NR | Yes (parotid gland) |
| Bedrosian et al. | 1 | M/61 | NA | Left | Mass | 9 | Submaxilectomy | No |
| Our institution | 1 | F/74 | 11 | Right (homolateral) | Painless mass | 50 × 25 × 20 | submaxilectomy | Yes (peritoneum, minor omentum, left suprarenal gland) |
M, male; F, female; NA, not applicable; NR, not reported; CMT, chemotherapy; RDT, radiotherapy.
With regard to the diagnostic process, it is valid to order an FNAB to make an etiologic diagnosis due to its low cost and high efficiency [4]. It is important to make differential diagnosis with primary tumors of the salivary glands (Table 2) [8–10].
Table 2.
Differential diagnosis of submandibular gland tumors with clear cells
| Differential diagnosis | Histopathology | Immunohistochemistry |
|---|---|---|
| Acinic cell carcinoma | Multiple cell types (in order of frequency): serous acinar, intercalated duct type, vacuolated, non-specific glandular and clear cells. The clear cell component shows pale non-staining cytoplasm, very similar in size and shape to the acinar type cells without glycogen. | Non-specific |
| Mucoepidermoid carcinoma | Malignant epithelial tumor with variable components of mucous, epidermoid and intermediate cells. Clear cell variant usually <10% of cells but can be a dominant finding, may contain glycogen or mucin. | Positive for p63, CK5/6, HER2 and negative for: GFAP, S100, CK20 (In the non-clear cell component) |
| Oncocytoma | Clear cell oncocytes with glycogen accumulation that shows an organoid architectural arrangement similar as conventional oncocytomas. Conventional eosinophilic oncocytes sometimes can be found interspersed with clear cells. |
|
| Myoepithelial carcinoma | Neoplastic myoepithelial cells with variable morphology: plasmacytoid, epithelioid, clear cell or spindled and infiltrative growth pattern. | Positive for CK, EMA, S100, SMA, calponin, p63, GFAP |
| Clear cell carcinoma | Malignant cells with clear cytoplasm with distinct cell borders, arranged in nests surrounded by sclerotic or hyalinized stroma and variable fibrocellular myxoid stroma. | Positive for CK AE1/AE3, p63 and negative for S100, SMA, Calponin, CK20, GFAP |
| CCRCC metastasis | Solid, alveolar, acinar, cystic growth patterns of cells with clear or eosinophilic cytoplasm and a characteristic network of small, thin walled vasculature. | Positive for CK AE1/AE3, vimentin, CAIX,, RCCC marker, CD10, PAX 8. Negative for CEA, SMA, GFAP, p63 and S100 |
CK, Cytokeratin; HER2, human epidermal growth factor 2; EMA, antiendomysial antibody; PAX 8, paired-box gene 8; CEA, carcynoembrionic antigen; S100, S-100 protein; CD10, cluster of differentiation 10; p63, protein 63; RCCC, clear cell renal cell carcinoma; SMA, smooth muscle actin; GFAP, glial fibrillary acid protein; CAIX, carbonic anhydrase IX.
The treatment given to each patient depended on the stage of the illness at the moment of diagnosis. In five out of eight cases a submandibulectomy was performed, and one case received chemotherapy followed by radiotherapy. There is no data about the treatment performed in the other two cases. Our patient received neoadjuvant treatment with pazopanib for 1 year, and due to the lack of response, a surgical approach was taken, performing cytorreduction and a right submandibulectomy.
In conclusion, RCCC metastases to the submandibular gland are extremely rare.
Finding a submandibular mass makes it necessary to rule out primary malignancies and HN metastasis in the first stage. However, in patients with a history of RCCC, metastasis from this localization should also be considered as a differential diagnosis.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
CONSENT
Consent was obtained from the patient for publication.
ETHICAL APPROVAL
Ethical approval and informed consent were obtained and are available upon request.
AVAILABILITY OF DATA AND MATERIALS
Data are available from the Institution’s medical record. The data cannot be publicly available due to local legal restrictions.
REFERENCES
- 1. Miah MS, White SJ, Oommen G, Birney E, Majumdar S. Late simultaneous metastasis of renal cell carcinoma to the submandibular and thyroid glands seven years after radical nephrectomy. Int J Otolaryngol 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moudouni SM, Tligui M, Doublet JD, Haab F, Gattegno B, Thibault P. Late metastasis of renal cell carcinoma to the submaxillary gland 10 years after radical nephrectomy. Int J Urol 2006;13:431–2. [DOI] [PubMed] [Google Scholar]
- 3. Balaban M, Dogruyol SV, Idilman IS, Unal O, Ipek A. Renal cell carcinoma metastasis to ipsilateral parotid and submandibular glands: report of a case with sonoelastographic findings. Pol J Radiol 2016;81:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serouya SM, Dultz LA, Concors SJ, Wang B, Patel KN. Late solitary metastasis of renal cell carcinoma to the submandibular gland. J Oral Maxillofac Surg 2012;70:2356–9. [DOI] [PubMed] [Google Scholar]
- 5. Majewska H, Skálová A, Radecka K, Stodulski D, Hyrcza M, Stankiewicz C, et al. . Renal clear cell carcinoma metastasis to salivary glands—a series of 9 cases: clinico-pathological study. Pol J Pathol 2016;1:39–45. [DOI] [PubMed] [Google Scholar]
- 6. Ficarra G, Pierleoni L, Panzoni E. Metastatic renal cell carcinoma involving Wharton’s duct: a case report. Oral Surg Oral Med Oral Pathol Oral Radio Endod 1996;81:580. [DOI] [PubMed] [Google Scholar]
- 7. Montie JE. Follow-up after partial or total nephrectomy for renal cell carcinoma In: Resnick MI, ed. The Urologic Clinics North America. Recurrent Malignant Disease. Philadelphia: Saunders, 1994;590. [PubMed] [Google Scholar]
- 8. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumors. 4th edn Lyon: IARC, 2017. [Google Scholar]
- 9. Ellis GL, Auclair PL. Tumors of the Salivary Glands: Atlas of Tumor Pathology. 3rd edn AFIP, 1996. [Google Scholar]
- 10. Thompson LDR, Wenig BM. Diagnostic Pathology: Head and Neck. 2nd edn Elsevier, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Institution’s medical record. The data cannot be publicly available due to local legal restrictions.



