Figure 4.
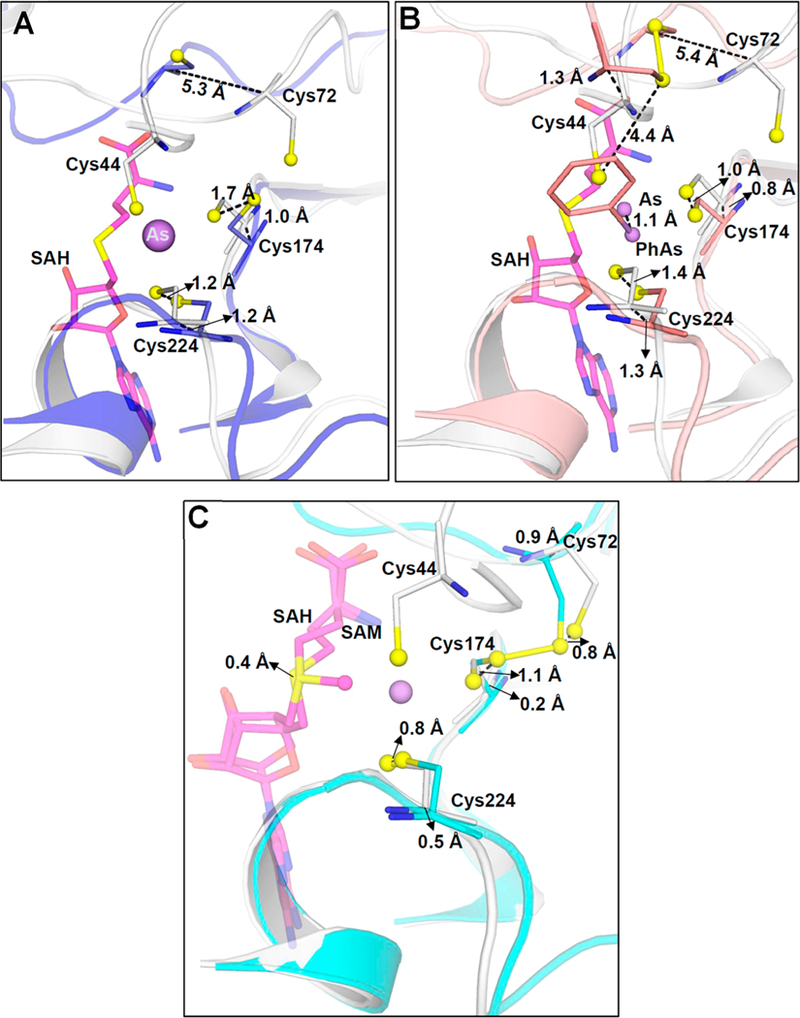
Movement of conserved cysteine thiols during substrate and/or product binding. (A) Superimposition of As(III)/SAH-bound CmArsM (gray) (PDB entry 6CX6) with unliganded CmArsM (blue) (PDB entry 4FS8) shows that the loop containing Cys72 moves 5.3 Å toward the As binding site when As(III) and SAH are bound. (B) Superimposition of As(III)/SAH-bound CmArsM (gray) with PhAs(III)-bound CmArsM (salmon) (PDB entry 4KW7) shows that the loop moves 5.4 Å in the direction of the As binding site relative to the site with bound PhAs(III). (C) Superimposition of As(III)/SAH-bound CmArsM (gray) with SAM-bound CmArsM (cyan) (PDB entry 4FR0) shows that the loop is 0.9 Å closer to the As binding site when SAH and As(III) are bound compared to when SAM is bound. Conserved cysteine residues, SAM, and SAH are represented as balls and sticks. Cα−Cα distances are indicated.
