Abstract
Background:
Dementia with Lewy bodies is characterized by transient clinical features, including fluctuating cognition and visual hallucinations, implicating dysfunction of cerebral hub regions, such as the pulvinar nuclei of the thalamus. However, the pulvinar is typically only mildly affected by Lewy body pathology in dementia with Lewy bodies, suggesting additional factors may account for its proposed dysfunction.
Methods:
We conducted a comprehensive analysis of postmortem pulvinar tissue using whole-transcriptome RNA sequencing, protein expression analysis, and histological evaluation.
Results:
We identified 321 transcripts as significantly different between dementia with Lewy bodies cases and neurologically normal controls, with gene ontology pathway analysis suggesting the enrichment of transcripts related to synapses and positive regulation of immune functioning. At the protein level, proteins related to synaptic efficiency were decreased, and general synaptic markers remained intact. Analysis of glial subpopulations revealed astrogliosis without activated microglia, which was associated with synaptic changes but not neurodegenerative pathology.
Discussion:
These results indicate that the pulvinar, a region with relatively low Lewy body pathological burden, manifests changes at the molecular level that differ from previous reports in a more severely affected region. We speculate that these alterations result from neurodegenerative changes in regions connected to the pulvinar and likely contribute to a variety of cognitive changes resulting from decreased cortical synchrony in dementia with Lewy bodies.
Keywords: Lewy, dementia, pulvinar, RNA, glia
Dementia with Lewy bodies (DLB) is thought to be the second most common form of neurodegenerative dementia after Alzheimer’s disease (AD).1 Clinically, DLB is marked by 4 core symptoms of fluctuating cognition, parkinsonism, visual hallucinations, and rapid eye movement sleep behavior disorder, against the backdrop of global cognitive decline.2 Pathologically, DLB is characterized by pathological aggregates of α-synuclein in nerve cell bodies and nerve cells processes termed Lewy bodies and Lewy neurites, respectively.3 However, varying degrees of AD-type pathology, consisting of extracellular amyloid-β plaques and intraneuronal tangles of abnormally hyperphosphorylated tau, are frequent concomitant features.4,5
Visuo-perceptual and attentional functions are impaired in DLB6–8 and may promote the occurrence of visual hallucinations.9–12 The pulvinar contributes to visuo-perceptual and attentional functions,13 has reciprocal connectivity with widespread cortical regions,14 and is a putative “hub” that coordinates neural activity across the cortex.15 Dysfunction of highly interconnected hubs has been postulated as important in eliciting the clinical manifestation of neurodegenerative disorders, including DLB, by diminishing network coherence and coordinated neural activity.16 Although most research on network connectivity in neurodegenerative disorders has focused on AD,17 connectivity is decreased to a greater degree in DLB compared to AD, with particular impairments in long-distance connections.18
Metabolic deficits19 and increased tissue diffusivity20 have previously been reported in the pulvinar in DLB. We have previously reported neuronal loss in the pulvinar, which may promote attentional dysfunction and visual hallucinations in DLB.21 However, Lewy body pathology is relatively mild in the pulvinar22 and the subregions most severely affected by α-synuclein aggregation did not show neuronal loss.21 Therefore, it is difficult to relate the myriad changes described previously in the pulvinar with the manifest burden of α-synuclein pathology. On that basis, we have investigated differential gene expression with whole-transcriptome RNA sequencing (RNA-seq), protein quantification assays, and histological analysis to evaluate changes to the pulvinar that may be relevant to the clinical features of DLB.
Methods
Tissue Preparation
All tissue was obtained from Newcastle Brain Tissue Resource, a U.K. Human Tissue Authority–approved research tissue repository, and ethical approval was granted by Newcastle University Ethics Board and the Joint Ethics Committee of Newcastle and North Tyne-side Health Authority (reference 08/H0906/136). DLB cases had been part of prospective clinical studies and had received detailed clinical assessments during life and case note review after death. All cases had consented to the use of their brain tissue for research purposes. Neuropathological assessment was conducted according to standardized neuropathological diagnostic procedures.4,23–26 Clinical and pathological data were collated to establish a clinico-pathological consensus diagnosis. The present study included cases with a clinical diagnosis of DLB confirmed by neuro-pathological postmortem assessment. DLB cases were compared to aged individuals with an absence of neurological features intra vitam and low age-associated neurodegenerative pathology. Demographic information is provided in Supplementary Tables 1 and 2.
At autopsy, tissue from the left hemisphere was cut into 1-cm thick coronal sections and rapidly frozen at −80°C between copper blocks. The pulvinar was identified by its location in the posterior pole of the thalamus from which approximately 50 mg of tissue was dissected with a cooled scalpel.27 Frozen tissue was obtained from a cohort of 15 control and 14 DLB cases (Supplementary Tables 1).
The right hemisphere was fixed in 10% formalin and dissected into blocks for neuropathological assessment. 10-μm sections were taken from the pulvinar at the level of the posterior aspect of the lateral geniculate nucleus and the amygdala and stained with antibodies against a range of protein targets using Menarini Mena-path Polymer detection kits (Menarini, Berkshire, UK) and counterstained with haematoxylin. Fixed pulvinar tissue was obtained from a cohort of 14 controls and 14 DLB cases (Supplementary Table 2).
RNA Isolation and Sequencing
Frozen tissue was placed in 5 to 10 volumes of pre-cooled RNAlater solution (Ambion, Warrington, UK) and stored at −80°C. Tissue was removed from RNA-later and rapidly homogenized in TRI™-reagent (Ambion) and stored at −80°C. RNA was extracted using a spin column method, as per the manufacturer’s instructions (Ribopure; Ambion), and 1 μg of RNA was DNase-treated (Turbo-DNase free; Ambion). The RNA concentration was determined using a Nanodrop ND 1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USASanta Cruz: Dallas, TX, USA) and the RNA integrity number was examined with an Agilent 2100 Bioanalyzer RNA 6000 Nano Assay (Agilent Technologies, Stockport, UK).
RNA-seq libraries were prepared using TruSeq Ribo Zero Gold kits (Illumina, San Diego, California, USA). Clustering was performed with 10 nM libraries pooled in groups of 6 libraries per lane of each flow cell. We then sequenced 200 bp paired-end libraries on a HiSeq2500 sequencer (Illumina, San Diego, California, USA). Sequence reads were aligned using Salmon. Genes with low expression (row mean counts for <1) were removed, and differential expression was estimated using DESEQ228 using the following model to correct for biological correlates:
Within DESEQ2, P values for differential expression from Wald tests were corrected for multiple testing using the Benjamini-Hochberg false discovery rate approach, with significant results reported at α =.05. Gene ontology (GO) enrichment was performed using gProfileR.29
Transcriptomic changes were evaluated at the protein level using western blot analysis (Supplementary Protocol 1).
Microscopy
To quantify glial subpopulations and neuropatho-logical lesions in the pulvinar in a separate cohort of cases and α-synuclein pathology in the amygdala of the cases used for the transcriptomic study, images were taken on a Zeiss AxioVision Z.1 microscope using a DsFi1 camera (Nikon, Japan). As detailed previously,21,30 Stereologer software was used to delineate a region of interest with a 2.5 × objective, prior to the placement of disector frames in a uniform, random arrangement. This method prevented the introduction of bias by giving every area of the region of interest an equal probability of being sampled for analysis. Disector frame sizes were determined based on the size of the measured particles and their distribution across the region of interest. In all cases, amyloid-b (4G8 anti-amyloid-β, 1:15000; Covance, Princeton, New Jersey, USA) was analyzed using 10 × objective and α-synuclein (5G4 anti-α-synuclein, 1:4500; Analytik, Jena, Germany) and tau (AT8 anti-tau, 1:4000; Auto-gen, Holliston, Massachusetts, USA); the microglial markers HLA-DP/DQ/DR (CR3/43, 1:1000; Dako, Glostrup, Denmark), CD74 (LN-2, 1:500; Santa Cruz, Dallas, TX, USA), and Iba1 (1:1000; Wako, Osaka, Japan); and the astrocytic markers glial fibrillary acidic protein (GFAP) (Z0334, 1:10000; Dako, Denmark) and aldehyde dehydrogenase family member 1 (ALDH1L1) (N103/39, 1:7500; Millipore, Billerica, Massachusetts, USA) were measured using 20 × objective.
We determined the percentage area occupied by individual antibodies by analyzing images by determining red-green-blue thresholds using the ImagePro Plus v.4.1 image analysis system (Media Cybernetics, Bethesda, Maryland). Size restriction was used with the 4G8 antibody to ensure that intracellular amyloid-b was not included in the analysis. In addition to quantitative analysis, we qualitatively assessed Iba1 morphology as described previously.31 We also qualitatively determined the presence of Alzheimer type II astrocytes, the histopathological hallmark of manganism and hepatic encephalopathy,32 as their presence was noted in a substantial number of cases.
These findings were correlated with densitometric analyses of neuropathological lesion burden to evaluate whether neuroglial marker expression was related to pathological protein deposition. A subset of cases used for histological analysis (8/14 control; 8/14 DLB) had been assessed as part of a previous stereological study of the pulvinar.21 Therefore, we additionally included stereological determination of total neuronal number within these analyses.
Results
Demographic Data
Demographic data for the RNA-seq and protein expression analysis cohort is shown in Supplementary Tables 1. There was no significant difference between groups in age at death (t = 0.18, df = 22, P=.862), postmortem interval (t = 0.17, df = 22, P = .863), and, where available, tissue pH (t = 0.60, df = 15, P = .555). There was no significant difference in the proportion of males relative to females between DLB and control (χ2 = 2.10, df = 1, P=.148). However, the groups were slightly unbalanced with 9/11 DLB males and 7/13 controls male. Braak neurofibrillary tangle (NFT) stage was significantly higher in DLB subjects when compared with controls (t = 3.85, df = 19, P=.001).
Demographic data for the histological analysis cohort are shown in Supplementary Table 2. There was no significant difference between groups in age at death (t = 0.0.23, df = 26, P = .982) or postmortem interval (t = 1.23, df = 26, P=.217). There was no significant difference in the proportion of males relative to females between DLB cases and controls (χ2 = 0.57, df = 1, P = .706). Braak NFT was significantly higher in the DLB cases when compared with the controls (t = 3.88, df = 26, P=.001).
Nomination of Differential Pulvinar Gene Expression Between DLB and Controls by RNA Sequencing
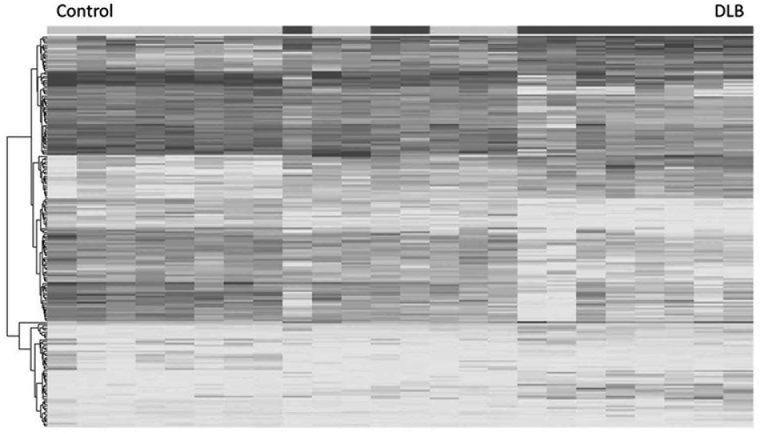
Our RNA-seq analysis revealed a partial separation between the DLB cases and controls in overall gene expression (Fig. 1). Quality control data is included in the supplementary QC file. From this analysis, we nominated 321 transcripts significantly different between controls and DLB cases after correction for multiple testing. Subsequently, GO enrichment analysis demonstrated several pathways were enriched in DLB cases when compared with controls (Table 1). We focused on genes related to synapses (GO:0045202, p 5 1.75E-25) and positive regulation of immune system process (GO:0002684, p = 7.75E-22).
FIG. 1.
Heat map showing separation between control and dementia with Lewy bodies (DLB) cases, with some degree of overlap. As illustrated, changes broadly mapped onto 2 gene ontology clusters, synapses, and positive regulators of immune system process.
TABLE 1.
Top 20 gene ontology enrichment
| p value | Term size | Overlap size | Recall | Precision | Term ID | Domain | Subgraph number | Term name |
|---|---|---|---|---|---|---|---|---|
| 2.05E-29 | 5658 | 483 | 0.442 | 0.085 | G0:0051179 | BP | 2 | Localization |
| 1.62E-28 | 5444 | 467 | 0.427 | 0.086 | G0:0048518 | BP | 2 | Positive regulation of biological process |
| 2.27E-28 | 6288 | 518 | 0.474 | 0.082 | G0:0044700 | BP | 2 | Single organism signalling |
| 3.36E-28 | 6297 | 518 | 0.474 | 0.082 | G0:0023052 | BP | 2 | Signalling |
| 4.69E-27 | 6392 | 520 | 0.476 | 0.081 | G0:0007154 | BP | 2 | Cell communications |
| 1.75E-25 | 691 | 114 | 0.104 | 0.165 | G0:0045202 | CC | 29 | Synapse |
| 2.31E-25 | 3537 | 334 | 0.306 | 0.094 | G0:0031988 | CC | 29 | Membrane-bound vesicle |
| 2.92E-25 | 4685 | 409 | 0.374 | 0.087 | G0:0048522 | BP | 2 | Positive regulation of cellular process |
| 3.42E-24 | 3664 | 339 | 0.310 | 0.093 | G0:0031982 | CC | 29 | Vesicle |
| 7.78E-24 | 10688 | 747 | 0.683 | 0.070 | G0:0005515 | MF | 158 | Protein binding |
| 2.81E-23 | 2325 | 243 | 0.222 | 0.105 | G0:0032879 | BP | 2 | Regulation of localization |
| 6.17E-23 | 4103 | 364 | 0.333 | 0.089 | G0:1902578 | BP | 2 | Single-organism localisation |
| 1.56E-22 | 4637 | 397 | 0.363 | 0.086 | G0:0051234 | BP | 2 | Establishment of localisation |
| 1.81E-22 | 3565 | 327 | 0.299 | 0.092 | G0:0065008 | BP | 2 | Regulation of biological |
| 3.19E-22 | 4512 | 388 | 0.355 | 0.086 | G0:0006810 | BP | 2 | Transport |
| 3.45E-22 | 2788 | 273 | 0.250 | 0.098 | G0:0007166 | BP | 2 | Cell surface receptor signalling pathway |
| 5.78E-22 | 1051 | 140 | 0.128 | 0.133 | G0:0009611 | BP | 2 | Response to wounding |
| 7.75E-22 | 939 | 130 | 0.119 | 0.138 | G0:0002684 | BP | 2 | Positive regulation of immune system process |
| 1.21E-21 | 3663 | 331 | 0.303 | 0.090 | G0:0048583 | BP | 2 | Regulation of response to stimulus |
| 1.26E-21 | 1190 | 151 | 0.138 | 0.127 | G0:0097458 | CC | 29 | Neuron part |
Data in bold signify the pathways chosen for further analysis.
Validation of Synaptic and Immune Proteins by Western Blot Analysis
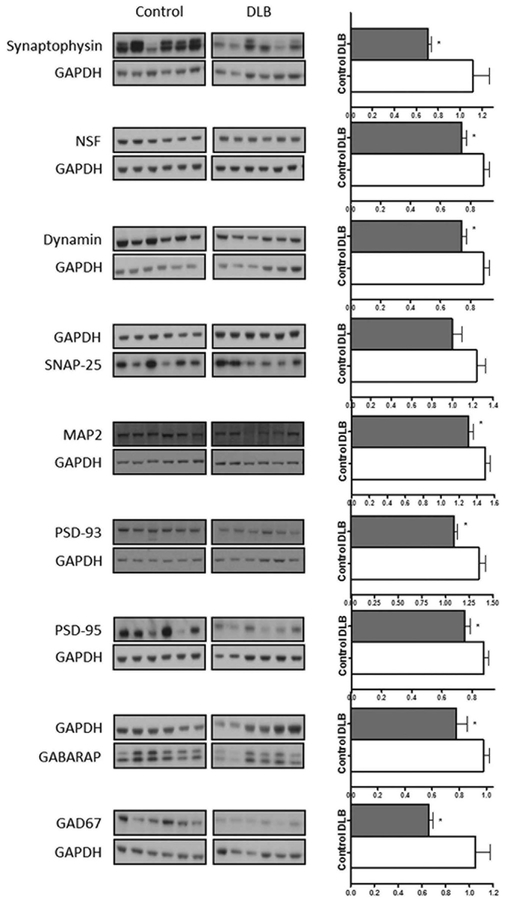
Analysis of protein expression using western blot analysis of general presynaptic markers demonstrated a significantly lower expression of synaptophysin (U = 30, P = .015), N-ethylmalemide sensitive fusion protein (NSF) (U = 25.5, P = .006), and dynamin (U = 37.5, P = .047) in DLB cases when compared with controls (Fig. 2). This was consistent with RNA-seq data, which demonstrated a significantly lower expression of SYP (P = .01), NSF (P = .01), and DNM1 (P = .03). However, no significant differences were found in syntaxin 1A (STX1A), synaptopsomal-associated protein 25 (SNAP25), synaptic vesicle glycoprotein 2B (SV2B), or growth associated protein 43 (GAP43), despite a significantly lower expression at the mRNA level.
FIG. 2.
Representative western blots of synaptic protein markers in dementia with Lewy (DLB) bodies when compared with control and graphs showing target protein band size and intensity normalized to GAPDH. ⋆P <.05. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NSF, N-ethylmalemide sensitive fusion protein; MAP2, microtubule associated protein 2; PSD-93, post synaptic density protein 93; GAD67, glutamic acid decarboxylase.
Analysis of protein expression using western blot analysis of general postsynaptic markers identified a significantly lower expression of the dendritic marker microtubule associated protein 2 (MAP2) (U = 35, P = .034) in DLB cases when compared with controls (Fig. 2), consistent with lower MAP2 mRNA (P = .04). The excitatory synaptic markers post synaptic density protein 93 (PSD-93) (U = 25.5, P = .011) and post synaptic density protein 95 (PSD-95) (U = 27, P = .009) were also lower in DLB cases when compared with controls (Fig. 2), consistent with reductions in DLG3 (P < .01) and DLG4 (P < .01) mRNA.
The inhibitory synaptic marker gamma aminobutyric acid receptor associated protein (GABARAP) (U=37, P = .046) was significantly reduced in DLB cases when compared with controls (Fig. 2), consistent with a reduction in GABARAP mRNA (P = .04). However, the protein levels of the inhibitory postsynaptic marker gephyrin were not significantly lower in the DLB cases when compared with the controls, despite being lower at the mRNA level. The GABA-ergic neuron marker glutamic acid decarboxylase 67 (GAD67) was lower in the DLB cases when compared with the controls on western blot (U = 32, P = .022) and also at the mRNA level (P = .02; Fig. 2).
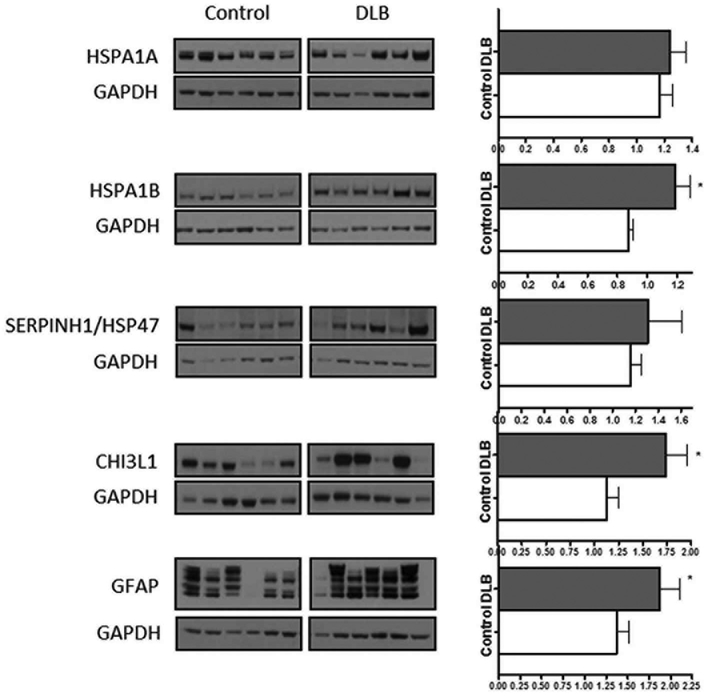
Analyses of chitinase 3-like 1 (CHI3L1), a positive regulator of immune system process and a proinflammatory marker,33 demonstrated significantly higher protein levels (U = 35, P = .034; Fig. 3). The astrocytic marker GFAP was also higher in the DLB cases relative to control (U = 37, P = .046; Fig. 3). heat shock protein 70 1B (HSPA1B) was significantly increased in the DLB cases compared with the controls (U = 22, P = .003). However, SERPINH1/HSP47 and heat shock protein 70 1A (HSPA1A) were not significantly different in the DLB cases when compared with the control cases (Fig. 3), despite showing differences for the same marker in RNA-seq.
FIG. 3.
Representative western blot images of immune markers in dementia with Lewy bodies when compared with control, and graphs showing target protein band size and intensity normalized to GAPDH. ⋆P <.05. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HSPA1A, heat shock protein 70 1A; HSPA1B, heat shock protein 70 1B; CHI3L1, chitinase 3-like 1, GFAP - glial fibrillary acidic protein.
Microscopy
As RNA-seq demonstrated an increase in transcripts related to positive regulation of the immune system process, we histologically assessed markers of micro-glia and astrocytes, the resident immune cells of the brain, in a separate cohort of DLB and control cases. We assessed the expression of the cytotoxic M1 micro-glial markers CD74 and HLA-DP/DQ/DR, the general microglial marker Iba1, and the astrocytic markers ALDH1L1 and GFAP in the pulvinar of DLB cases compared with controls. We also assessed α-synuclein, amyloid-β, and tau expression to evaluate whether immune cell expression was related to the presence of neurodegenerative pathologies.
The observed Lewy body pathology was greater than that previously reported in another study of the pulvinar in DLB, which described an absence of Lewy bodies but sparse neuritic pathology.22 This discrepancy may be the result of our use of the 5G4 antibody, which is reported to show more widespread asynuclein pathology.34 Nevertheless, Lewy bodies were not frequently encountered within the pulvinar of most cases, with Lewy body burden typically corresponding to absent or mild deposition under previously described semiquantitative assessment methods.4 However, we noted an abundance of asynuclein immunoreactive dots and occasional fine threads, as noted previously with the 5G4 antibody.35
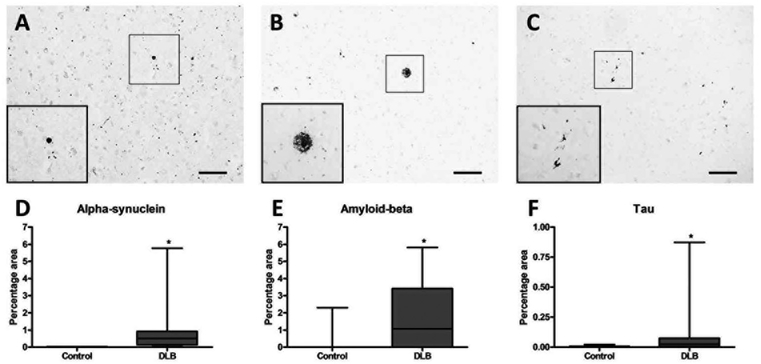
α-synuclein (U = 0, P < .001), amyloid-β (U = 39, P = .006), and tau (U = 37, P = .004) were higher in the pulvinar of the DLB cases when compared with those of the controls (Fig. 4). Although AIF1 mRNA was significantly elevated in the DLB pulvinar on RNA-seq (P = .003), its protein product Iba1 was not increased on histological analysis (Supplementary Fig. 1). Similarly, CD74 mRNA was significantly higher in DLB (P = .02), but was not different on histological analysis (Supplementary Fig. 1). Although some specific subtypes of HLA-D were significantly increased at the mRNA level, there was no significant difference in the expression of HLA-DP/DQ/DR on histological analysis (Supplementary Fig. 1). The astrocytic marker ALDH1L1 was not significantly different on RNA-seq or histological analysis between DLB cases and controls. However, GFAP was significantly higher in the DLB cases when compared with controls at the mRNA level (P = .001) and on histological analysis (U = 18, P = .001; Supplementary Fig. 1).
FIG. 4.
Photomicrographs demonstrating a-synuclein (A), amyloid-β (B), and tau (C) pathology in the pulvinar. Densitometric analysis demonstrated α-synuclein (D), amyloid-β (E), and tau (F) were significantly higher in dementia with Lewy bodies when compared with control. Scale bar (A) = 50 μm and inset is 2x the original image. Scale bar (B & C) = 100 μm and inset are 4 × the original images.
A range of different microglial morphologies were observed across cases and within experimental groups (Supplementary Fig. 2). Possibly as a result of the considerable heterogeneity in morphologies within groups, no morphology was significantly associated with either experimental group (χ2 = 4.5, P = .214; Supplementary Fig. 3). Furthermore, individual microglial morphologies were not associated with any histopathological or glial marker. Alzheimer type-II astrocytes were not more frequently encountered in the DLB cases when compared with the controls (χ2 5 2.8, P = .104; Supplementary Fig. 3).
Relationship Between Synaptic Loss and Neuropathological Changes
To evaluate whether synaptic loss corresponded to neuropathological changes in a region projecting to the pulvinar, we quantified the burden of α-synuclein pathology in the amygdala, a region connected to the pulvinar through a pathway reported to be dysfunctional in DLB.20 Of the 9 synaptic markers significantly reduced in DLB when cases compared with controls, only PSD-93 was significantly negatively correlated with a-synuclein burden in the amygdala (rs = 20.729, P = .017).
Relationship Between Astrocytic Increases and Neuropathological, Stereological, and Synaptic Changes
After identifying an increase in GFAP in DLB cases compared with controls, we next evaluated the relation of this marker to the presence of neuropathological lesions, neuronal loss, and synaptic changes. To prevent spurious correlations being identified because of group differences, DLB cases were analyzed separately from controls. The histological expression of GFAP was not significantly related to amyloid-β, tau, or α-synuclein in the DLB cases. Within the subset of cases assessed using stereological determination of neuronal number (8/14) as reported previously,21 GFAP was not related to neuronal number.
Employing 2 distinct cohorts of cases for transcriptomic and histological analyses limited our ability to compare histologically assessed glial markers and synaptic markers assessed with western blot. Therefore, we also assessed GFAP using western blot analysis to investigate whether GFAP expression was related to synaptic changes in DLB. GFAP (50 kDa) was significantly negatively correlated with synaptophysin (rs = −0.621, P = .041), dynamin (rs = −0.655, P = .029), GABARAP (rs = −0.673, P = .023), and GAP43 (rs = −0.627, P = .039) in the DLB cases.
Clinico-Pathological Correlations
A subset of DLB cases (9/14) used for histological analysis had been subject to neuropsychological evaluation intra vitam. As detailed previously,30 these individuals had been assessed using the hallucinations subscale of the Neuropsychiatric Inventory (NPI) within 2 years prior to death.36 Comparison of the NPI hallucination score with neuropathological markers and GFAP demonstrated a significant positive correlation only between tau burden and NPI hallucination subscale score (rs = 0.701, P = .035). There were no significant correlations between the NPI hallucination subscale and any other variable.
Discussion
Using a transcriptomic approach, the present study demonstrated synaptic changes and astrogliosis in the pulvinar in DLB. Notably, these findings occurred in a region that typically manifests relatively mild asynuclein deposition yet is postulated to play a central role in the cognitive profile of DLB. The reported changes differ markedly from a previous study that employed RNA-seq in the cingulate gyrus, a region with more severe α-synuclein pathology,2 and that reported genes involved in neurogenesis and myelination enriched in DLB cases when compared with controls.37
The reported synaptic changes indicate lower expression levels of presynaptic proteins such as synaptophysin and NSF, which support efficient turnover of vesicles following exocytotic events.38 In contrast, we found the preservation of proteins necessary for vesicular exocytosis, such as SNAP25,39 STX1A,40 and SV2B.41 Despite the interaction of α-synuclein with synaptic proteins, previous studies have not consistently demonstrated significantly lower levels of pre-synaptic markers in DLB.42
The role of glia in DLB has been a matter of controversy and debate, with conflicting reports in the literature. Microglial activation is induced by aggregated asynuclein in vitro,43 although postmortem studies have reported inconsistent findings.31,44–46 Despite RNA-seq demonstrating enrichment of transcripts related to the positive regulation of immune system processes, we found no evidence of such changes at the protein level. Therefore, our data favor the view that microglia-mediated neuroinflammatory processes are not an important factor in the reported synaptic changes. However, it is impossible to exclude the possibility that an acute inflammatory response occurred earlier in the disease process but was undetectable in terminal stages.
As GFAP immunoreactivity did not correlate with any pathological lesion or neuronal loss, astrogliosis does not seem to be a response to neurodegenerative lesions within the pulvinar. It is noteworthy that astrogliosis was not accompanied by microgliosis and thus does not appear to signify a neuroinflammatory state. Considering the negative relationship between reactive astrogliosis and several synaptic markers, we speculate that reactive astrogliosis may be a response to synaptic dysfunction, with the aim of supporting synaptic transmission. Further studies are warranted to evaluate the role of astrocytes in Lewy body diseases and whether they have a protective or degenerative function. Elucidating the role of astrocytic subpopulations in neurodegenerative disorders may identify novel therapeutic targets to augment protective functions or attenuate degenerative processes.
The role of the pulvinar as a “hub” modulating cortico-cortical activity may suggest that the present findings are the neuropathological substrate of desynchronous network coherence in DLB. The pulvinar exerts a powerful influence on cortical activity based on attentional demands,47 meaning its dysfunction likely impacts attention-mediated cortical functions. Attention is deficient in DLB48,49 and has been implicated in visual hallucinations and fluctuating cognition.11,48 The search for the neuropathological sub-strates of symptoms such as visual hallucinations and cognitive fluctuations is impeded by the inherent difficulty in attributing a transient feature to a permanent neuropathological change. However, the dysfunction of structures regulating cortical functioning on the basis of attention may be more likely to contribute to transient features of neurodegenerative diseases.
The reported findings are within a region with relatively low levels of Lewy body pathology and differ from those reported in a more severely affected region, the cingulate gyrus.37 These findings indicate important molecular changes, in addition to previously reported neuronal loss,21 independent of the severity of local neuropathological changes. Although we noted a relationship between tau pathology in the pulvinar and the frequency and severity of visual hallucinations intra vitam, the overall levels of tau were very low in the pulvinar in DLB. Furthermore, these findings are hard to reconcile with our previous report of higher tau burdens in the pulvinar of AD cases without visual hallucinations compared to DLB.50 The tau burden in the pulvinar may be a proxy measure of global tau burden, which has been previously reported to influence the clinical phenotype of DLB.51
As the pulvinar is highly interconnected with numerous cortical and subcortical areas, one may speculate that the reported findings are a downstream result of neuropathological changes to regions connected to the pulvinar. We identified a strong negative correlation between PSD-93 and α-synuclein burden in the amygdala, a region connected to the pulvinar. Although a similar relationship was not found with other synaptic markers, the pulvinar as a “hub” region has widespread connectivity across the cortex,13 and a systematic evaluation of the many regions connected to it was beyond the scope of this study. Molecular changes in “preserved” regions as a result of neuropathological changes elsewhere may be particularly relevant to the aetiopathology of Lewy body diseases, considering the relatively selective topography of α-synuclein deposition.52 Therefore, relative preservation of brain structures may have important implications for the clinical phenotype of DLB, and studies focusing only on regions with severe α-synuclein deposition may miss pathological alterations relevant to the clinical pheno-type of Lewy body disease.
In summary, we identified changes on the molecular level in the pulvinar, a region with relatively low levels of Lewy body pathology but that is thought to have an important influence on the cognitive phenotype of DLB.13 One may speculate that the reported synaptic and astroglial changes are a downstream effect of neurodegenerative changes elsewhere and suggest that the absence of a significant local pathological burden should not be assumed to indicate functional preservation.
Supplementary Material
Acknowledgments:
The authors wish to express their gratitude to the individuals who kindly donated their brain tissue to Newcastle Brain Tissue Resource. The technical assistance of Mary Johnson is also gratefully acknowledged. Tissue for this study was provided by Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK Medical Research Council (G0400074), by NIHR Newcastle Biomedical Research Centre and Unit awarded to the Newcastle upon Tyne NHS Foundation Trust and Newcastle University, and as part of the Brains for Dementia Research Program jointly funded by Alzheimer’s Research UK and Alzheimer’s Society.
Funding agencies:
This study was funded by the National Health Service (NHS) National Institute of Health Research Biomedical Research Unit for Lewy body dementia at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, from the Yvonne Emily Mairy bequest. This research was also supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging.
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
References
- 1.Heidebrink JL. Is dementia with Lewy bodies the second most common cause of dementia? J Geriatr Psychiatry Neurol 2002; 15(4):182–187. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium [published online ahead of print June 9, 2017]. Neurology.
- 3.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature 1997; 388(6645):839–840. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65(12):1863–1872. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017;16(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshizawa H, Vonsattel JP, Honig LS. Early neuropsychological discriminants for Lewy body disease: an autopsy series. J Neurol Neurosurg Psychiatry 2013;84(12):1326–1330. [DOI] [PubMed] [Google Scholar]
- 7.Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL. Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. Alzheimer Dis Assoc Disord 2009;23(4): 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrova M, Mehrabian-Spasova S, Aarsland D, Raycheva M, Traykov L. Clinical and neuropsychological differences between mild Parkinson’s disease dementia and dementia with Lewy bodies. Dement Geriatr Cogn Dis Extra 2015;5(2):212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collerton D, Perry E, McKeith L. Why people see things that are not there: A novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci 2005; 28(6):737. [DOI] [PubMed] [Google Scholar]
- 10.Meppelink AM, Koerts J, Borg M, Leenders KL, van Laar T. Visual object recognition and attention in Parkinson’s disease patients with visual hallucinations. Mov Disord 2008;23(13):1906–1912. [DOI] [PubMed] [Google Scholar]
- 11.Cagnin A, Gnoato F, Jelcic N, et al. Clinical and cognitive correlates of visual hallucinations in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2013;84(5):505–510. [DOI] [PubMed] [Google Scholar]
- 12.Hall JM, O’Callaghan C, Shine JM, et al. Dysfunction in attentional processing in patients with Parkinson’s disease and visual hallucinations. J Neural Transm (Vienna) 2016;123(5):503–507. [DOI] [PubMed] [Google Scholar]
- 13.Benarroch EE. Pulvinar: associative role in cortical function and clinical correlations. Neurology 2015;84(7):738–747. [DOI] [PubMed] [Google Scholar]
- 14.Leh SE, Chakravarty MM, Ptito A. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. Int J Biomed Imaging 2008;2008:789539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science (New York, NY) 2012;337(6095):753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol 2011;10(9):829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Chen Z, Gong G, Evans A. Neuronal networks in Alzheimer’s disease. Neuroscientist 2009;15(4):333–350. [DOI] [PubMed] [Google Scholar]
- 18.Peraza LR, Taylor JP, Kaiser M. Divergent brain functional network alterations in dementia with Lewy bodies and Alzheimer’s disease. Neurobiol Aging 2015;36(9):2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perneczky R, Haussermann P, Diehl-Schmid J, et al. Metabolic correlates of brain reserve in dementia with Lewy bodies: an FDG PET study. Dement Geriatr Cogn Disord 2007;23(6):416–422. [DOI] [PubMed] [Google Scholar]
- 20.Delli Pizzi S, Maruotti V, Taylor JP, et al. Relevance of subcortical visual pathways disruption to visual symptoms in dementia with Lewy bodies. Cortex 2014;59:12–21. [DOI] [PubMed] [Google Scholar]
- 21.Erskine D, Thomas AJ, Attems J, et al. Specific patterns of neuronal loss in the pulvinar nucleus in dementia with lewy bodies. Mov Disord 2017;32(3):414–422. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto R, Iseki E, Murayama N, et al. Investigation of Lewy pathology in the visual pathway of brains of dementia with Lewy bodies. J Neurol Sci 2006;246(1–2):95–101. [DOI] [PubMed] [Google Scholar]
- 23.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58(12):1791–800. [DOI] [PubMed] [Google Scholar]
- 25.Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology 1995;45(3 Pt 1):461–466. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Muller CM, Rub U, et al. Pathology associated with sporadic Parkinson’s disease—where does it end? J Neural Trans 2006(70):89–97. [DOI] [PubMed] [Google Scholar]
- 27.Jones EG. The Thalamus. Springer Science & Business Media; 2012. Cambridge, UK. [Google Scholar]
- 28.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reimand J, Arak T, Adler P, et al. g:Profiler-a web server for functional interpretation of gene lists (2016 update). Nucleic Acid Res 2016;44(W1):W83–W89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erskine D, Thomas AJ, Taylor J-P, et al. Neuronal loss and asynuclein pathology in the superior colliculus and its relationship to visual hallucinations in dementia with Lewy bodies. Am J Geriat Psych 2017;25(6):595–604. [DOI] [PubMed] [Google Scholar]
- 31.Bachstetter AD, Van Eldik LJ, Schmitt FA, et al. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Comm 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norenberg MD. The role of astrocytes in hepatic encephalopathy. Neurochem Pathol 1987;6(1–2):13–33. [DOI] [PubMed] [Google Scholar]
- 33.Wennstrom M, Surova Y, Hall S, et al. The inflammatory marker YKL-40 is elevated in cerebrospinal fluid from patients with Alzheimer’s but not Parkinson’s disease or dementia with Lewy bodies. PloS One 2015;10(8):e0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs GG, Wagner U, Dumont B, et al. An antibody with high reactivity for disease-associated alpha-synuclein reveals extensive brain pathology. Acta Neuropathol 2012;124(1):37–50. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs GG, Breydo L, Green R, et al. Intracellular processing of disease-associated alpha-synuclein in the human brain suggests prion-like cell-to-cell spread. Neurobiol Dis 2014;69:76–92. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 37.Pietrzak M, Papp A, Curtis A, et al. Gene expression profiling of brain samples from patients with Lewy body dementia. Biochem Biophys Res Commun 2016;479(4):875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M, Wu S, Zhou Q, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature 2015;518(7537):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 1998;395(6700):347–353. [DOI] [PubMed] [Google Scholar]
- 40.Mishima T, Fujiwara T, Kofuji T, Akagawa K. Impairment of catecholamine systems during induction of long-term potentiation at hippocampal CA1 synapses in HPC-1/syntaxin 1A knock-out mice. J Neurosci 2012;32(1):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem 2004; 279(50):52124–52131. [DOI] [PubMed] [Google Scholar]
- 42.Vallortigara J, Whitfield D, Quelch W, et al. Decreased levels of VAMP2 and Monomeric alpha-synuclein correlate with duration of dementia. J Alzheimers Dis 2016;50(1):101–110. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. Faseb J 2005;19(6):533–542. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd CE, Thiel E, McCann H, Harding AJ, Halliday GM. Cortical inflammation in Alzheimer disease but not dementia with Lewy bodies. Arch Neurol 2000;57(6):817–822. [DOI] [PubMed] [Google Scholar]
- 45.Mackenzie IR. Activated microglia in dementia with Lewy bodies. Neurology 2000;55(1):132–134. [DOI] [PubMed] [Google Scholar]
- 46.Streit WJ, Xue QS. Microglia in dementia with Lewy bodies. Brain Behav Immun 2016;55:191–201. [DOI] [PubMed] [Google Scholar]
- 47.Zhou H, Schafer RJ, Desimone R. Pulvinar-cortex interactions in vision and attention. Neuron 2016;89(1):209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballard C, O’Brien J, Gray A, et al. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol 2001;58(6):977–982. [DOI] [PubMed] [Google Scholar]
- 49.Peters F, Ergis AM, Gauthier S, et al. Abnormal temporal dynamics of visual attention in Alzheimer’s disease and in dementia with Lewy bodies. Neurobiol Aging 2012;33(5):1012 e1–e10. [DOI] [PubMed] [Google Scholar]
- 50.Erskine D, Thomas AJ, Attems J, et al. Specific patterns of neuronal loss in the pulvinar nucleus in dementia with lewy bodies. Mov Disord 2017;32(3):414–422. [DOI] [PubMed] [Google Scholar]
- 51.Gomperts SN, Locascio JJ, Makaretz SJ, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol 2016;73(11):1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 2017;18(2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.