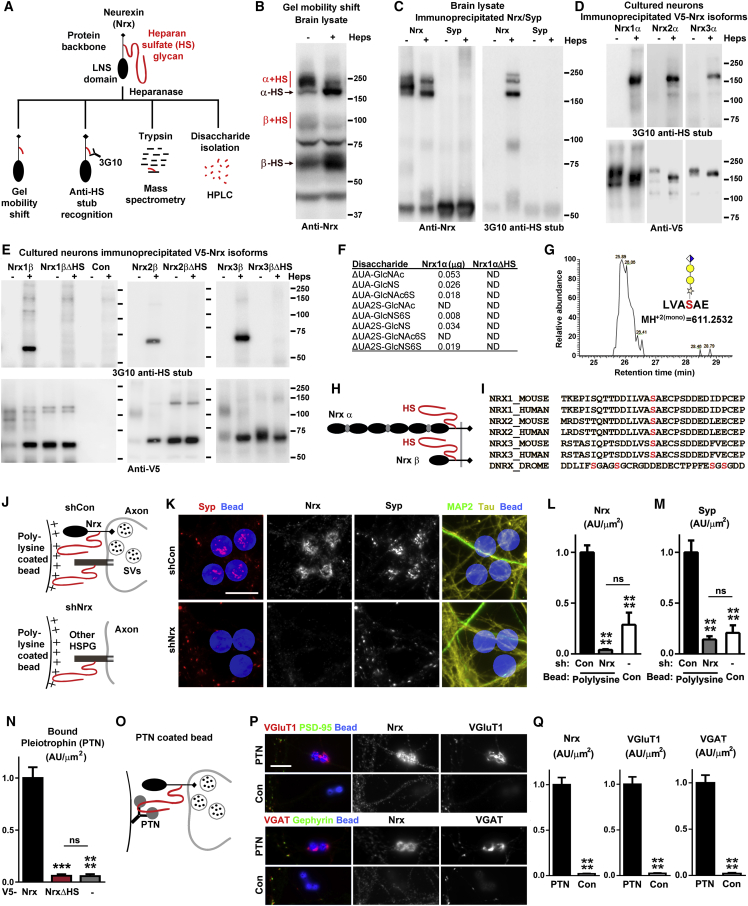
Figure 1.
Nrxs Are HSPGs and Mediate Presynaptic Induction by HS Binding Factors
(A) Schematic outline for identification of Nrxs as HSPGs.
(B) Native Nrx shows molecular weight shifts upon cleavage of HS with heparinase (Heps). Prominent Nrx α and β bands are indicated.
(C) Immunoprecipitated native Nrx shows molecular weight shifts with heparinase and is recognized by an HS antibody; 3G10 recognizes the HS stub remaining after heparinase cleavage (intact HS is not well recognized by western blot and the heparinase treatment helps condense the bands to improve detection). Anti-synaptophysin (syp) was used as a control for immunoprecipitation.
(D) All recombinant V5-tagged α Nrx expressed in neurons show molecular weight shifts with heparinase and are recognized by an HS antibody.
(E) Point mutation of each V5-tagged β Nrx lacking splice inserts confirms the HS modification site in neurons; Nrx1,2,3βΔHS Ser→Ala mutants lack HS modification; Con used empty vector. The untreated V5-Nrxβ signal may appear diffuse due to modification with HS chains of varying length; the signal collapses to one band upon heparinase treatment. These assays were done in neurons as Nrx is poorly modified in cell lines (Figure S1B).
(F) HPLC following heparinase cleavage confirms the presence of HS disaccharides in purified Nrx1α but not Nrx1αΔHS ectodomain. ΔUA, Δ4,5-unsaturated uronic acid; GlcNAc, N-acetylglucosamine; GlcNS, N-sulfoglucosamine; 2S, 2-O-sulfation; 6S, 6-O-sulfation; ND, not detected.
(G) Identification of HS modified peptide from Nrx1α recombinant protein. Selected Isotopic Chromatograph of m/z 611.2532 (10 ppm mass tolerance) corresponding to the glycopeptide ‘LVAS(Pentose-Hexose-Hexose-Uronic Acid)AE’ from NRx1α, which is consistent with the Xylose-Galactose-Galactose-Glucuronic Acid tetrasaccharide that would remain attached to the protein backbone of an HSPG following digestion with heparinase. The fragmentation ions from this glycopeptide were weak in intensity, most likely due to the poor modification of Nrx in HEK293 cells and the relative complexity of the starting material coupled with the necessity to remove all disaccharides to allow for ionization and detection by nanospray MS. No signal was detected within 20 ppm mass tolerance corresponding to ‘LVAS(Pentose-Hexose-Hexose-Uronic Acid)AE’ or ‘LVAA(Pentose-Hexose-Hexose-Uronic Acid)AE’ from NRx1αΔHS, consistent with this mutation abolishing HS modification.
(H and I) The position of the conserved HS modification in Nrx is shown on the red serine in this sequence between the LNS and transmembrane domains, as identified in Figures S1C, S1E and in (E) and (G); for Dnrx, we did not distinguish whether all or a subset of these serines are modified.
(J–M) Clustering of presynaptic marker synaptophysin (Syp), a component of synaptic vesicles (SVs), and of Nrx induced by polylysine-coated beads was reduced by Nrx knockdown (shNrx) in comparison to control (shCon) at or below levels associated with uncoated (Con) beads. Measures are integrated intensity of puncta per contact area of beads with tau-positive axons lacking contact with microtubule-associated protein 2 (MAP2)-positive dendrites to exclude native synapses (AU, arbitrary units). ∗∗∗∗p < 0.0001 by Kruskal-Wallis and Dunn’s tests compared to shCon polylysine beads, n = 18–33 cells or 16–30 beads from 2–3 independent experiments.
(N) Recombinant pleiotrophin (PTN) bound to immature neurons expressing V5-Nrx, but not V5-NrxΔHS. Immature neurons were used because Nrx is not well modified in cell lines and the low levels of native Nrx were insufficient to mediate detectable binding to untransfected cells. ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 by Kruskal-Wallis and Dunn’s tests compared to Nrx, n = 17–38 cells from 2 independent experiments.
(O–Q) Beads coated with pleiotrophin induced clustering of native Nrx with vesicular glutamate transporter VGluT1 or with vesicular GABA transporter VGAT in contacting axons at sites lacking postsynaptic PSD-95 or gephyrin, respectively. Measures are integrated intensity of Nrx, VGluT1, or VGAT per bead area lacking postsynaptic markers. ∗∗∗∗p < 0.0001 by Mann-Whitney test, n = 27–33 beads from 2 independent experiments.
Western blot results are representative of two (C) or three (B, D, E) biological replicates. Error bars represent SEM. Scale bars: (K) 10 μm, (P) 20 μm. See also Figures S1 and S2 and Tables S1 and S2.