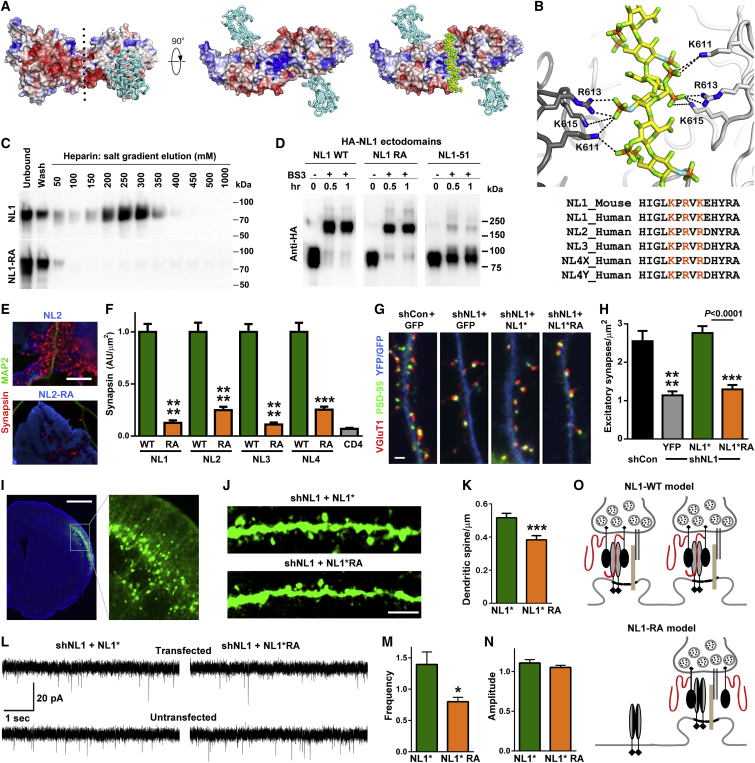
Figure 5.
NL Binding to HS Is Required for its Synapse Promoting Activity
(A) The NL1-Nrx1β LNS domain complex (PDB: 3VKF), side view. The NL1 surface is colored according to the electrostatic potential from red (−8 kbT/ec) to blue (+8 kbT/ec), the Nrx LNS domain is in aquamarine cartoon representation. Dotted line in the left panel indicates the 2-fold pseudo-symmetry axis of a NL1 dimer. The middle panel is rotated 90 degrees, corresponding to a view from the presynaptic side. This reveals a large basic surface lining the canyon formed between two NL1 molecules. A heparin dodecamer (PDB: 1HPN, shown in sphere representation, carbon atoms yellow; oxygen chartreuse) matches perfectly the dimensions of this canyon. Fitting was done manually.
(B) Close-up of possible interactions between docked heparin and basic residues lining the NL1 canyon (top), indicating the Arg or Lys residues mutated in this study and highlighted in orange in the sequences (bottom). Black dotted lines indicate putative hydrogen bonds.
(C) The NL1 ectodomain binds heparin, and binding is abolished by the RA mutation.
(D) The NL1 RA mutation does not interfere with its dimerization. Purified recombinant HA-tagged ectodomains of NL1 wild-type, RA mutant, and the −51 mutant reported to disrupt dimerization (Ko et al., 2009) were chemically cross-linked by treatment with 0.5 mM bis (sulfosuccinimidyl) suberate-d0 (BS3) for the indicated amounts of time.
(E and F) Presynaptic differentiation in contacting axons induced by each NL on COS7 cells was impaired by the RA mutations. ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 by Kruskal-Wallis and Dunn’s tests comparing NL RA with WT, n = 24–36 cells from 3 independent experiments. NL RA mutants did not differ significantly from CD4 control. Surface levels of NLs did not vary (Figure S5).
(G and H) Density of excitatory synapses (apposed PSD-95-VGluT1 puncta) in cultured hippocampal neurons was reduced by NL1 knockdown and rescued by RNAi-resistant NL1∗ but not NL1∗-RA. ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 by Kruskal-Wallis and Dunn’s tests, n = 31–39 cells from 3 independent experiments.
(I) NL1 knockdown and replacement with NL1∗ or NL1∗-RA plus GFP in mouse cortex by in utero electroporation.
(J and K) Spine density in layer 2/3 cortical neurons was reduced in NL1 knockdown cells expressing NL1∗-RA compared with NL1∗. ∗∗∗p < 0.001 by Mann-Whitney test, n = 33–34 dendrites from 3 mice each.
(L-N) mEPSCs were recorded from neighboring transfected and untransfected layer 2/3 cortical neurons. Data are presented as the ratio of average frequency or amplitude for transfected over untransfected cells. The NL1∗-RA group showed a reduction in frequency and amplitude compared with the NL1∗ group. ∗p < 0.05 by t test, n = 5–6 mice averaging from 2–9 cells per mouse. Individual cell data are shown in Figure S5.
(O) Model for NL1-containing synapses. NL1 binds to protein and HS domains of Nrx. Loss of HS binding by the NL1-RA mutation may result in loss of some synapses and maintenance of other synapses through NL1-Nrx protein domain interactions and additional synaptic organizing complexes.
Error bars represent SEM. Scale bar: (E) 10 μm, (G) 2 μm, (I) 1 mm, (J) 5 μm. See also Figure S5.