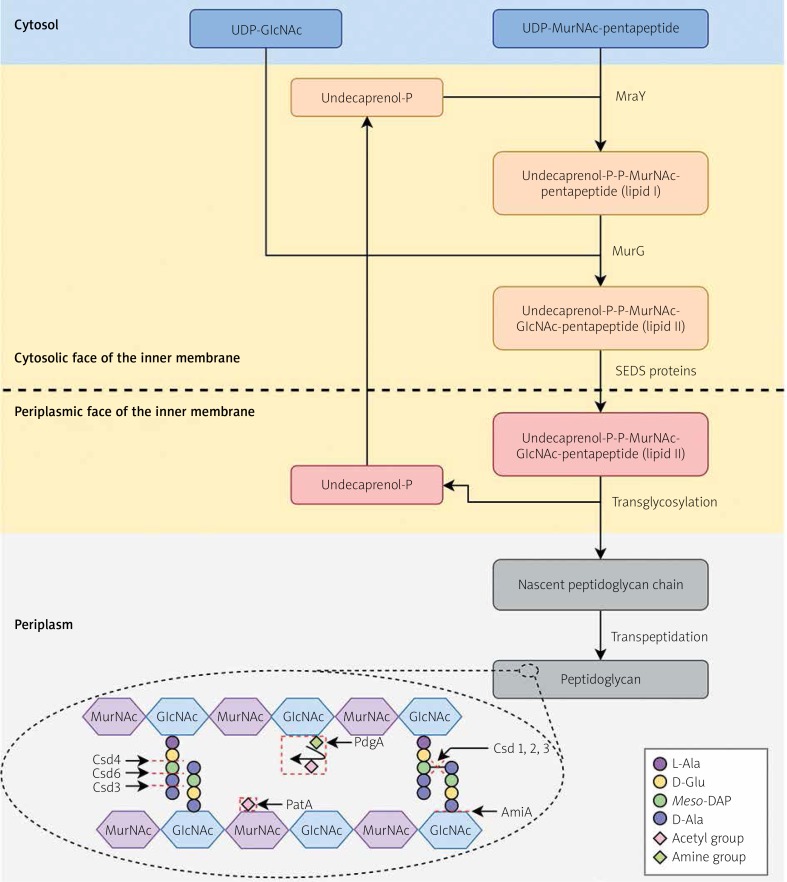
Figure 3.
Peptidoglycan biosynthesis and modifications in Helicobacter pylori. Within the cytoplasm, peptidoglycan precursors are synthesised, i.e. UDP-GlcNAc and UDP-N-MurNAc-pentapeptide. In the action of MraY, membrane-anchored lipid I is synthesised by linking UDP-N-MurNAc-pentapeptide to the lipid carrier, undecaprenyl phosphate. Then, UDP-GlcNAc is attached, which together with lipid I forms lipid II. This process is carried out by MurG. The formation of a complex with undecaprenyl phosphate allows the translocation of hydrophilic precursors by a hydrophobic inner membrane. The translocation of the complex occurs through the action of SEDS (shape, elongation, division, and sporulation) proteins. Such a molecule in the periplasmic environment is then biochemically processed by glycosyltransferases (linear polymerisation) and transpeptidases (peptide crosslinking). Undecaprenyl pyrophosphate undergoes dephosphorylation, which affects its availability and the possibility of carrying out subsequent translocations of peptidoglycan synthesis precursors. The Csd1, Csd2, and Csd3 proteins have D,D-endopeptidase activity, cleaving a 4-3 peptide bond (or DD-cross link) linking a muropeptide dimer. Furthermore, Csd3 also functions as a D,D-carboxypeptidase whose targets are pentapeptide monomers. The resulting GM-tetrapeptides are processed by Csd6, forming GM-tripeptides, subsequently trimmed to GM-dipeptides by the Csd4 activity. The AmiA, by an activity of N-acetylmuramoyl-L-alanyl amidase, cleavages the link between N-acetylmuramoyl residue and L-alanine. The PgdA and PatA lead to the N-deacetylation of N-acetylglucosamine and O-acetylation of N-acetylmuramic acid, respectively. Based on [63, 83] with minor modifications
L-Ala – L-alanine, D-Ala – D-alanine, meso-DAP – meso-diaminopimelic acid, D-Glu – D-glutamic acid, GlcNAc – N-acetylglucosamine, MurNAc – N-acetylmuramic acid.