Figure 1. Unique antibody-receptor exchange mechanism underlies TReg bias of mixed IL-2/JES6–1 complex.
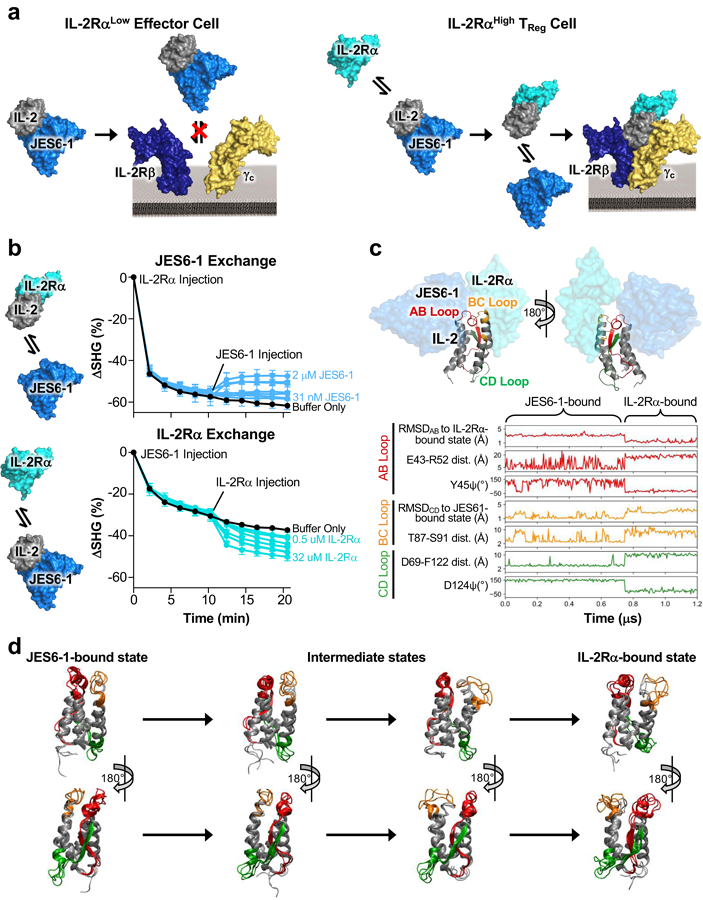
(a) Schematic of the mechanistic rationale for IL-2/JES6–1 complex-mediated selective potentiation of TReg cells. The JES6–1 antibody (shown in single-chain format) sterically obstructs IL-2 engagement of the IL-2Rβ and γc subunits, preventing activation of IL-2RαLow effector cells (left). However, allosteric exchange between JES6–1 and the IL-2Rα subunit allows for exclusive signaling on IL-2RαHigh TRegs, biasing toward an immunosuppressive response (right). (b) IL-2 was immobilized and 500 nM IL-2Rα (top) or 500 nM JES6–1 antibody (bottom) was injected at time 0 min. After 10 minutes, various concentrations of JES6–1 antibody ranging from 31 nM to 2 μM (top) or various concentrations of IL-2Rα ranging from 0.5 μM to 32 μM (bottom) were added and second-harmonic generation signal change (ΔSHG) was monitored. Exchange schemes are shown at left. (c) Molecular structure of the IL-2 cytokine bound to JES6–1 (PDB ID 4YQX) (17) overlaid with the IL-2Rα subunit from the IL-2 cytokine-receptor quaternary complex structure (PDB ID 2B5I) (3), highlighting the AB (red), BC (orange), and CD (green) interhelical loops of the cytokine (top). Molecular dynamics simulations of free IL-2 were conducted starting from the cytokine’s conformations in the crystallographic structures of IL-2 bound to the JES6–1 and S4B6 antibodies and the IL-2Rα subunit. Root mean square deviation (RMSD), dihedral angles, and inter-residue distances for the interhelical loops and flanking residues are plotted for a representative transition between the JES6–1-bound and IL-2Rα-bound states (bottom). (d) Overlay of three representative simulated conformations each from the JES6–1-bound state, the intermediate states, and the IL-2Rα-bound state of IL-2 that form the primary transition path, with interhelical regions colored as in (c).
