Figure 3. Disruption of antibody-cytokine affinity enhances immunocytokine activity on IL-2Rα+ cells.
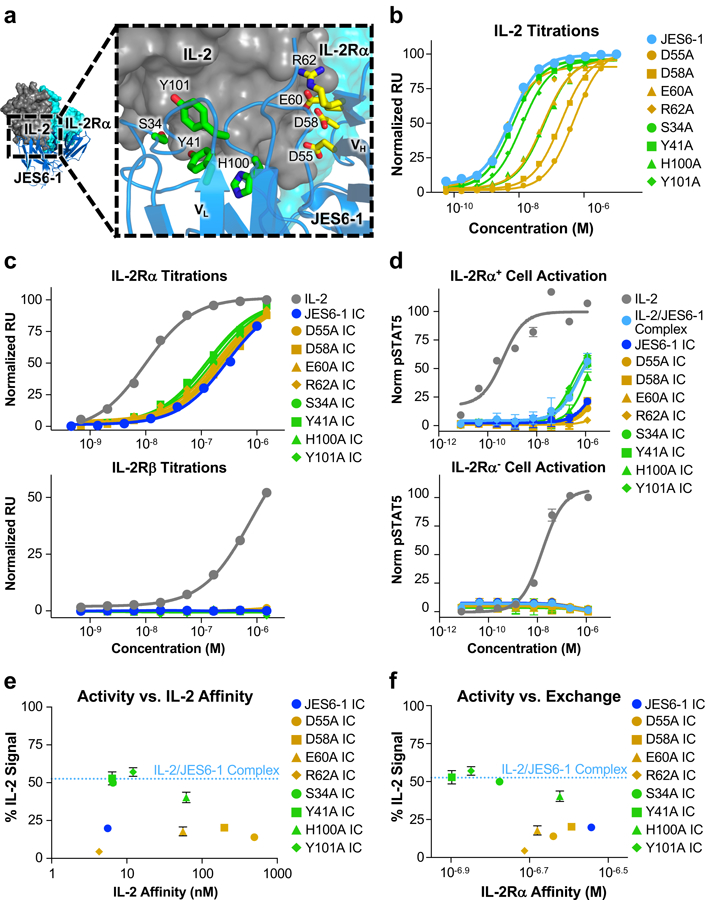
(a) Crystallographic structure of the IL-2/JES6–1 interface (PDB ID 4YQX) (17) with interfacial antibody residues that were mutated to alanine highlighted in yellow (heavy chain) or green (light chain). Human IL-2Rα is overlaid from the IL-2 cytokine-receptor quaternary complex structure for reference (PDB ID 2B5I) (3). (b) Equilibrium surface plasmon resonance titrations of soluble IL-2 binding to immobilized JES6–1 or the indicated antibody variants. (c) Equilibrium surface plasmon resonance titrations of soluble IL-2, JES6–1 IC, or JES6–1 IC mutants binding to immobilized IL-2Rα (top) or IL-2Rβ (bottom). (d) STAT5 phosphorylation response of IL-2Rα+ (top) or IL-2Rα− (bottom) YT-1 human NK cells treated with IL-2, JES6–1 IC, or JES6–1 IC mutants. Data represent mean ± s.d. (e) Comparison of the STAT5 phosphorylation activity of the indicated JES6–1 IC variants (% IL-2-induced signal at 1.2 μM concentration) versus IL-2 affinity of their corresponding antibodies. Activity of the IL-2/JES6–1 complex is indicated by the dashed blue line. Data represent mean ± s.d. (f) Comparison of the STAT5 phosphorylation activity of the indicated JES6–1 IC variants (% IL-2-induced signal at 1.2 μM concentration) to their IL-2Rα affinities (representative of their exchanging propensities). Activity of the IL-2/JES6–1 complex is indicated by the dashed blue line. Data represent mean ± s.d. Heavy chain mutations are colored yellow and light chain mutations are colored green throughout the figure.
