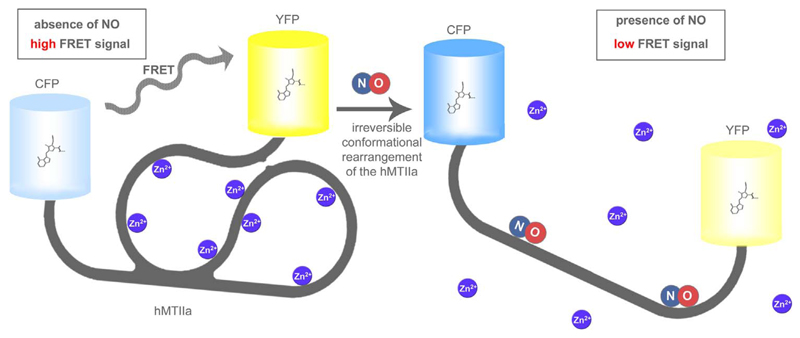
Fig. 4. Basic principle of FRET-MT.
In the absence of NO FRET-MT has a globular structure, which is stabilized by several Zn2+ ions yielding high FRET (left panel). NO binding to the MT domain yields a structural rearrangement. Thereby Zn2+ ions are released and the distance between the terminal CFP and YFP is significantly increased resulting in a decreased FRET ratio signal. The probe was mainly developed to confirm the interaction of NO with MT, but also used to measure NO in vitro and in living endothelial cells upon Ca2+ mobilization or the administration of NO and NO-donors.