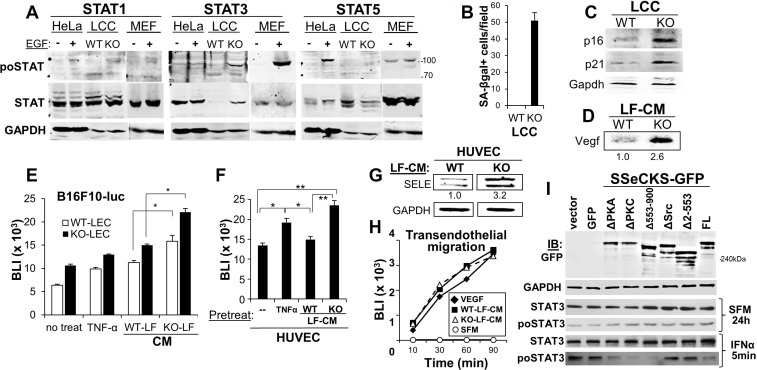
Figure 5. SSeCKS deficiency in lung fibroblasts results in premature senescence and SASP expression in vitro.
(A) Immunoblot analysis of relative Stat1, 3 or 5 phosphorylation (po- vs. total STAT) using lysates from hEGF-treated (100 ng/ml for 16 h) HeLa cells or mouse embryo fibroblast (MEF), or WT- or KO-LCC cells. GAPDH is assessed as a protein loading control. Protein molecular weight markers are shown at right. (B) Relative SA-βgal staining. Error bars, SEM from 6 independent microscopic fields, p < 0.001. Immunoblot analysis of WT- or KO-LCC lysates for p16, p21 or Gapdh (C) or LCM for secreted Vegf (D), quantified as described in Figure 4. (E) Adhesion of B16F10-luc on WT- or KO-LEC treated with serum-free media (SFM, “no treat”), TNF-α (16 h), or SFM containing 20% CM from WT- or KO-LF (2 h). Error bars, SEM of triplicate wells from three independent experiments. *p < 0.01. (F) Same adhesion experiment as in panel E using HUVEC monolayers. Error bars, SEM of triplicate wells from three independent experiments. *p < 0.01; **p < 0.001. (G) Immunoblot analysis of SELE and GAPDH expression in HUVEC and HMVEC-L incubated for 2 h with CM (20% total) from WT- or KO-LF, imaged and normalized as described in Figure 4. (H) B16F10-luc migration through HUVEC monolayers towards SFM supplemented with VEGF or CM (20%) from WT- or KO-LF. Migrating cells (isolated from the bottom of Boyden chamber membranes) were assessed by luciferase assay. (I) Two days after transfection with pcDNA3.1/neo plasmids expressing GFP or SSeCKS-GFP fusions (FL, full-length; Δ, SSeCKS domain deletion), KO-LEC were incubated for 2 d in G418 (400 μg/ml)-containing media, incubated for 24 h in SFM, then treated for 5 min with IFNα. Lysates were then assessed by immunoblot for GFP, GAPDH, total STAT3 or STAT3poY705. A representative blot is shown for two independent experiments.