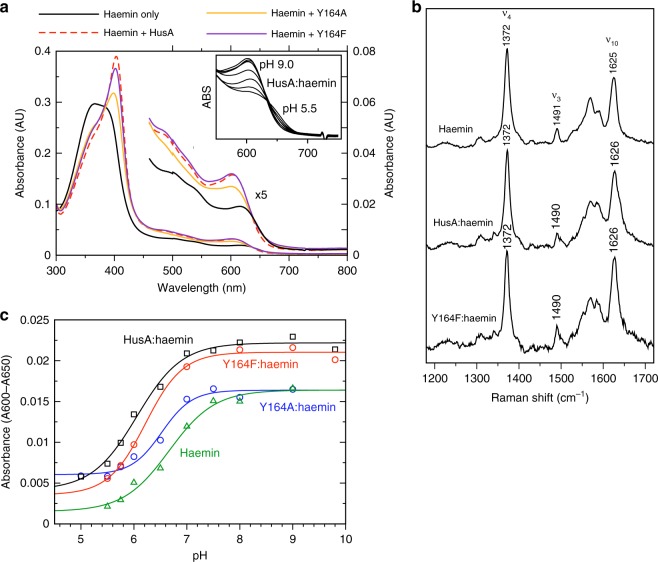
Fig. 3.
Spectroscopic studies suggest haem iron coordination is unchanged by HusA binding. a UV-visible spectra of 5-μM haemin in the presence of 7.5-µM HusA, Y164A and Y164F (0.2 M sodium phosphate, pH 7.0). Insert shows dependence of ~600 nm band on pH. b Resonance Raman spectra recorded with excitation at 488 nm for haemin (10 mM), HusA (3.2 mM) and HusA Y164F (3.5 mM). c Acid-alkaline transition of the absorbance band at ~600−650 nm for 5-µM haemin alone or bound with 7-µM HusA, Y164A or Y164F. Spectra were recorded in 0.1 M NaCl with 0.1 M of the following buffers: citrate, pH 5.0; BisTris-HCl, pH 5.5; BisTris-HCl, pH 5.75: BisTris-HCl, pH 6.0; sodium phosphate, pH 6.5; sodium phosphate, pH 7.0; sodium phosphate, pH 7.5; Tris-HCl, pH 8.0; sodium borate, pH 9.0; sodium borate/NaOH, pH 9.8; sodium carbonate, pH 10.0 (not all titration points were collected for each protein)