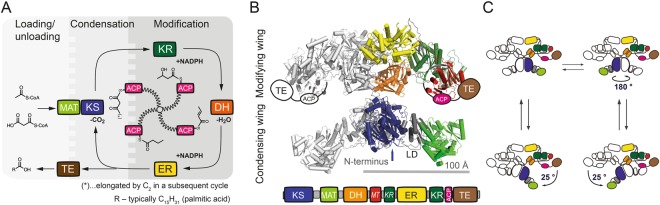
Figure 1.
Overview of animal fatty acid synthesis. (A) Fatty acid synthesis as occurring in animals. The fatty acid, typically palmitic acid, is produced from the substrates acetyl-CoA, malonyl-CoA and NADPH. The acetyl moiety is sequentially elongated and modified by several domains until a certain chain length (C16) is reached and the final product is released from the enzyme as a free fatty acid. During the whole process, all intermediates remain covalently attached to the enzyme, mainly to the ACP domain, which requires a high conformational freedom of FAS to facilitate productive interactions between the ACP domain and all catalytically active sites. Domain nomenclature: KS (ketoacyl synthase), KR (ketoacyl reductase), DH (dehydratase), ER (enoyl reductase), ACP (acyl carrier protein), TE (thioesterase), MAT (malonyl/acetyltransferase). (B) Cartoon depiction of the dimeric “X”-shaped structure of porcine FAS6. α-Helices are shown as cylinders. One half of the dimer is coloured according to the attached domain overview. Owing to their high positional variability, ACP and TE could not be traced in electron density, but are schematically drawn for clarity. KR and MT (methyltransferase) refer to non-catalytic folds, which have structural tasks and may confine the ACP during substrate shuttling. (C) Conformational dynamics of animal FAS. Swinging and swivelling motions around the flexible hinge region have been observed by single particle EM and high-speed atomic force microscopy8,15. Full rotation of the condensing wing by 180° was further confirmed by mutagenesis studies49.