Figure 2. Changes in dyspnea and wake disturbances in response to LVAD by implant strategy.
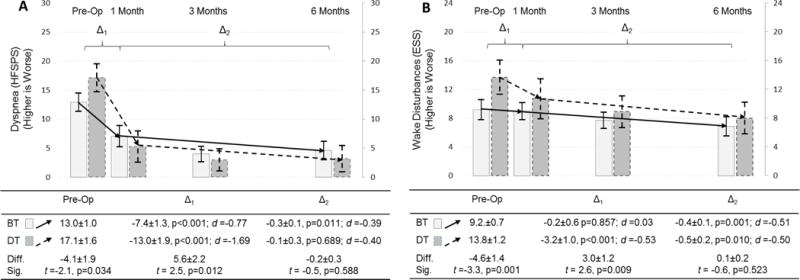
Changes in dyspnea (A; as measured by the Heart Failure Somatic Perception Scale dyspnea score) and wake disturbances (B; as measured by the Epworth Sleepiness Scale) are depicted comparing pre-operative assessment with measure taken at 1, 3 and 6 months after LVAD; the mean and 95% confidence interval is represented by column height and the high and low whisker bars, respectively. Solid lines and lighter bar graphs represent bridge therapy patients, and dashed lines and darker bars represent destination therapy patients. Two phases of change in symptoms are depicted; the initial response to LVAD between pre-implant and 1 month post-implant (Δ1), and the subsequent change between 1 and 6 months post-implant (Δ2). The effect size of each phase of change is also presented as Cohen’s d (d) (0.2–0.3 = small, ≈ 0.5 = moderate, and ≥ 0.8 = large effect). The difference (diff) and significance thereof (sig) of symptoms pre-implant and during each phase of change between destination and bridge therapy patients are presented below the figure.
Abbreviations: BT = bridge therapy; Diff. = difference; DT = destination therapy; ESS = Epworth Sleepiness Scale; HFSPS = Heart Failure Somatic Perception Scale; LVAD = left ventricular assist device; Sig. = significance; t = t-test.
