Fig. 4.
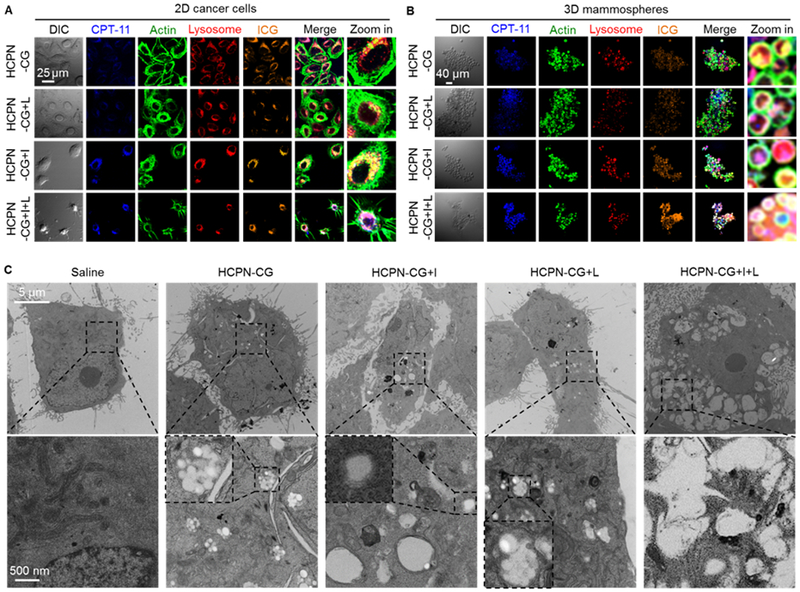
In vitro cell uptake of nanoparticles and their cold responsiveness inside cells. Confocal micrographs of (A) 2D cancer cells and (B) 3D mammosphere cells after incubating with HCPN-CG nanoparticles for 3 h at 37 °C, followed by cooling on ice (+I, 5 min), laser irradiation (+L, 1 W/cm2, 2 min), or both (+I+L). The cells incubated with HCPN-CG nanoparticles and further treated with ice cooling and laser irradiation (HCPN-CG+I+L) appear shrunken and/or spiky. (C) TEM images of cancer cells treated with saline, HCPN-CG nanoparticles alone, and HCPN-CG nanoparticles with ice cooling for 5 min (+I, 5 min), NIR laser irradiation for 2 min at 1 W/cm2 (+L, 1 W/cm2, 2 min), or both (+I+L). Endo/lysosomes can be easily observed as white dots in the low-magnification images (top row) in HCPN-CG treated cells (no such white dots in control cells treated with saline), due to the uptake of multiple HCPN-CG nanoparticles with a core-shell structure (Figure 2A) in the endo/lysosomes of the cells (bottom row). Importantly, almost all the nanoparticles disappeared/disassembled in the endo/lysosomes after ice cooling, but not NIR irradiation alone. The insets in the high-magnification images in the bottom row show endo/lysosomes either with intact HCPN-CG nanoparticles or without discernable nanoparticles (due to their disassembly upon ice cooling, +I). DIC: differential interference contrast.
