Figure 4.
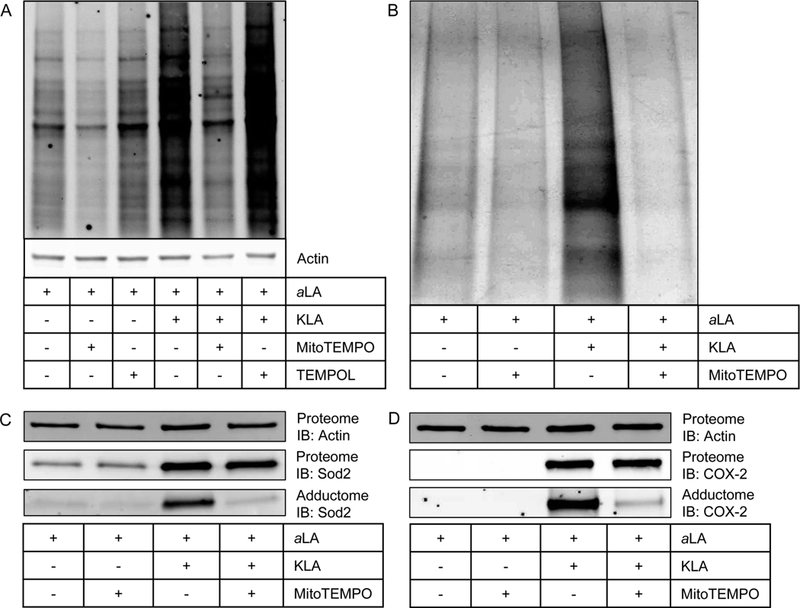
(A) Results of modulation of KLA-induced protein adduction by MitoTEMPO and TEMPOL. MitoTEMPO, a mitochondrially targeted superoxide scavenger, was able to reduce both physiological and pathophysiological protein adduction by lipid electrophiles compared to vehicle and KLA, respectively. TEMPOL, a ubiquitously dispersed superoxide scavenger, did not modulate electrophile formation in either physiological or pathophysiological settings. (B) Consistent with previous results, affinity-purified adducted protein is greatest in the KLA-activated macrophages. MitoTEMPO reduces total adducted protein in both the vehicle-treated and KLA-activated macrophages. (C) MitoTEMPO does not change the KLA-induced expression (Proteome) of Sod2 but does decrease the amount of Sod2 adduction (Adductome). (D) MitoTEMPO does not change the KLA-induced expression (Proteome) of Sod2 but does decrease the amount of Sod2 adduction (Adductome). These data indicate that both mitochondrial and nonmitochondrial targets are adducted by lipid electrophiles generated through a process that involves mitochondrial superoxide.
