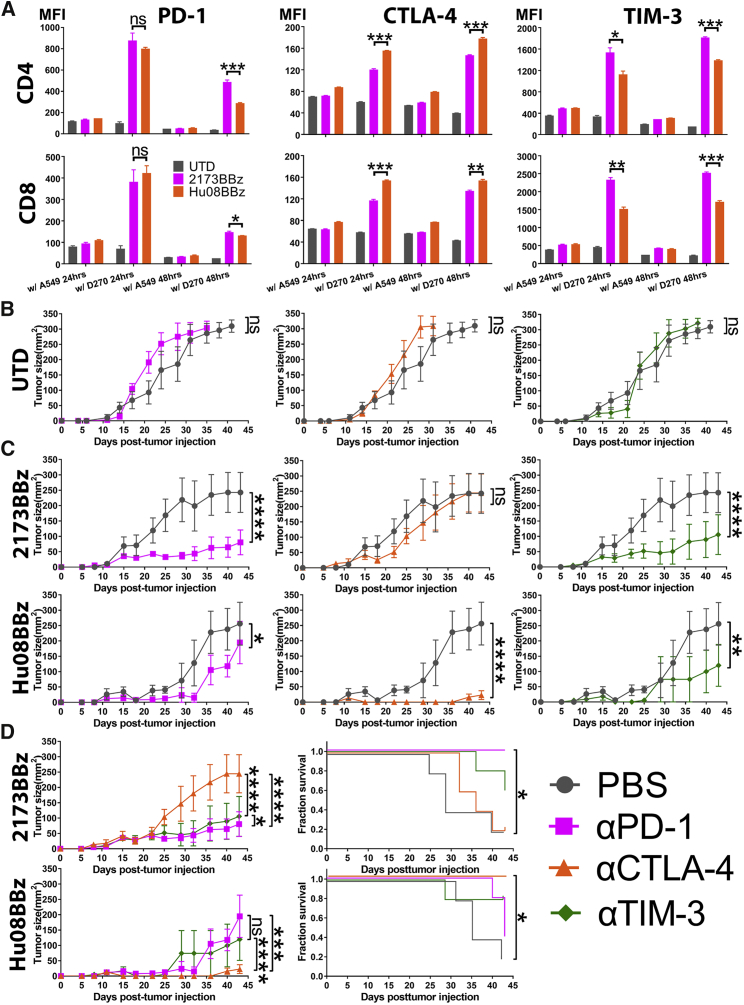
Figure 3.
Checkpoint Blockades Selectively Enhances the Function of CAR T Cells
(A) EGFRvIII- (2173BBz) targeting and IL-13Rα2- (Hu08BBz) targeting CAR T cells as well as un-transduced T cell control were co-cultured with target-positive D270 tumor cell line and target-negative A549 tumor cell line. The expression of checkpoint receptors on the T cells was determined by flow cytometry, by staining with fluorochrome-conjugated anti-checkpoint receptor antibodies; the median fluorescence intensity (MFI) was quantified on CD4 and CD8 CAR-positive T cells after 24- or 48-hr co-culture. Statistically significant differences were calculated by one-way ANOVA with post hoc Tukey test. (B) Un-transduced (UTD) human T cells were i.v. infused into a D270 subcutaneously implanted mouse model (n = 5 per group) 7 days after tumor implantation. From day 6, PBS or the same volume of 200 μg checkpoint blockade antibodies (anti-PD-1, anti-CTLA-4, and anti-TIM-3) were injected intraperitoneally every 4 days. Tumor size was measured and compared between the UTD plus PBS group and the UTD plus checkpoint blockade groups. (C) Same number of EGFRvIII-targeting (2173BBz) and IL-13Rα2-targeting (Hu08BBz) CAR T cells infused and combined with checkpoint blockade as described in (B). The tumor volume of checkpoint blockade combinational therapy groups was compared with PBS combined CAR T cell control group (n = 5 per group). (D) Different checkpoint blockade combinational therapies were compared in the EGFRvIII-targeting (2173BBz) and IL-13Rα2-targeting (Hu08BBz) CAR T cell groups based on the tumor size of mice. Survival curves were also compared in these two CAR T cell groups. Statistically significant differences of tumor growth between the experimental groups were determined by linear regression, and log-rank test was used for determining the statistically significant differences of survival curves. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data are presented as means ± SEM.